Pilomatrix Carcinoma: Case Report of a Rare Cutaneous Malignancy with Unique Reconstructive Considerations
Brandon K Richland, Khang T Nguyen, Vincent Laurence and Gregory RD Evans
DOI10.4172/2472-1905.100012
Brandon K Richland1*, Khang T Nguyen2, Vincent Laurence1 and Gregory RD Evans1
1Department of Plastic and Reconstructive Surgery, and Aesthetic & Plastic Surgery Institute, University of California Irvine Medical Center, Orange,CA, USA
2Northshore-Long Island Jewish Health System, USA
- *Corresponding Author:
- Brandon K Richland
Department of Plastic and Reconstructive Surgery and Aesthetic & Plastic Surgery Institute
University of California Irvine Medical Center
Orange, CA, USA.
Tel: 8583957649
Fax: 7144562229
E-mail: brichlan@uci.edu
Received date: February 08, 2016; Accepted date: February 28, 2016; Published date: March 10, 2016
Citation: Richland BK, Nguyen KT, Laurence V, et al. Pilomatrix Carcinoma: Case Report of a Rare Cutaneous Malignancy with Unique Reconstructive Considerations. J Aesthet Reconstr Surg. 2016, 2:1. doi: 10.4172/2472-1905.100012
Introduction
Pilomatrix carcinoma is a rare malignancy of the hair follicle characterized by aggressive local invasion, high rates of local recurrence, and metastasis in advanced stages. It is the malignant version of a pilomatrixoma. There is no consensus on optimal management, but current approaches typically involve wide local excision, sentinel lymph node biopsy and local reconstruction with skin grafting.
Case Report
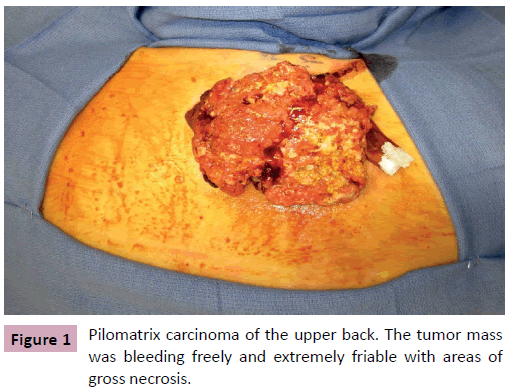
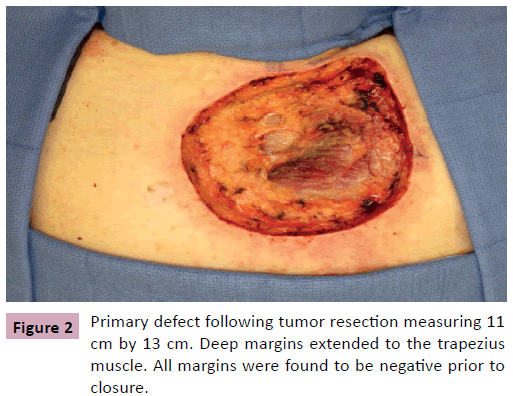
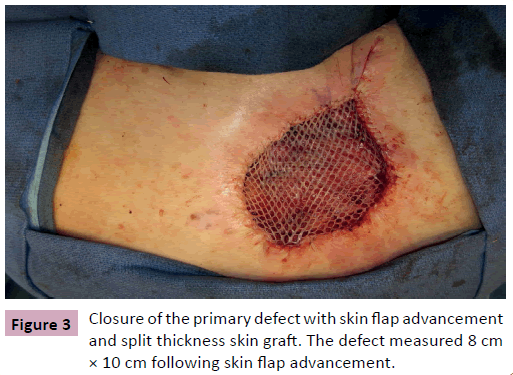
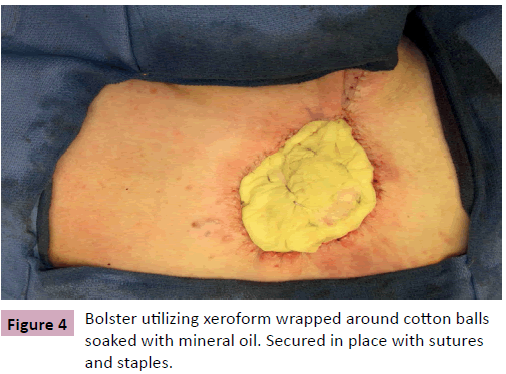
A 68 year-old male, presented for evaluation of an enlarging skin lesion on his right upper back over the past six years. On examination there was a 12 cm fungating, bleeding mass (Figure 1). Excisional biopsy histology revealed infiltrating atypical basaloid cells positive for beta-catenin immunostaining, consistent with pilomatrix carcinoma. Preoperatively, positron emission tomography (PET) demonstrated three nodes with mildly increased uptake: a left axillary node, an inferior left neck node, and a node immediately deep to the lesion. It was indeterminate by imaging whether the increased uptake was due to inflammation or metastatic cancer. Therefore intraoperative scintigraphy was performed that demonstrated greatest uptake at the axillary node. The surgical oncology team performed excisional biopsy of this sentinel node and wide local excision of the tumor with 2 cm circumferential margins and deep margins extending to the level of the trapezius muscle. All margins and the sentinel node were confirmed negative by frozen section prior to the plastic surgery team beginning our closure of the primary defect, which measured 11 cm × 13 cm (Figure 2). Skin flaps were undermined circumferentially and advanced to minimize the size of the defect. The trapezius muscle was released from its inferior margin and rotated to provide a well vascularized wound bed for skin grafting. The remaining 8 cm x 10 cm defect was repaired with split thickness skin graft from the posterior thigh and secured with a bolster dressing (Figures 3 and 4). More cosmetically satisfactory reconstructive options were considered, including rotational advancement, as well as pedicled versus free flap coverage, but given the very advanced nature of the lesion, the reported high rates of recurrence, and the significant likelihood of future biopsies / resections, we elected to pursue the simplest reconstructive option. At six months the patient was without recurrence and the skin graft had healed. There was a small step-off between the surrounding skin and skin graft but this was expected due to the depth of the original defect.
Discussion
Pilomatrix carcinoma is a rare malignancy of the hair follicle first described by Lopansri and Mihm in 1980 [1]. Cornejo and Deng found 101 cases reported in the English literature [2], while Melancon et al. identified an additional 34 cases in non-English literature [3]. The rarity of this malignancy makes prediction of incidence rates difficult; however, Sau et al. diagnosed pilomatrix carcinoma in only 20 of 1450 cases of pilar neoplasms [4]. Benign pilomatrixomas are relatively common in young girls, while the malignant form, pilomatrix carcinoma, is predominantly found in older males. There is a 4:1 male to female predominance and an average age of diagnosis at 45-51 years [3,4]. The distribution of pilomatrix carcinomas is overwhelmingly in the sun exposed areas of the skin, including the face (27%), neck (14%), scalp (10%), back (10%), arm or shoulder (10%), and leg (7%) [3,4].
The clinical course of pilomatrix carcinoma is often insidious and locally with reports of patients having this malignancy for over ten to twenty years before presentation [3,4]. Clinical diagnosis can be difficult as lesions may be mistaken for basal cell carcinoma, cutaneous cysts, lipomas and other cutaneous lesions [2-4]. Diagnosis relies on histology characterized by “proliferating basaloid cells arranged in sheets, irregular islands and bands” positive for beta-catenin immunostaining. Tumors can demonstrate highly variable degrees of anaplasia, high mitotic index, and keratinization. Full-thickness invasion of the dermis, subcutaneous fat, and underlying muscle fascia were noted in a number of specimens [4].
There is no clear consensus on the management of pilomatrix carcinoma, but non-operative management involving chemotherapy and radiation seems to have limited efficacy [5]. Surgical management is generally the standard. Recommended margins range from 0.5 to 3.0 cm due to the highly invasive nature of the disease [2,3]. Nevertheless, local recurrence rates remain high ranging from 59% to 64% in the first one to two years. The use of Mohs surgery has also been described [3], but an advantage in local recurrence is unclear given the limited power and followup time reported by the authors. The role of lymph node biopsy has not been studied despite a 10-16% rate of distant metastasis [2-4]. While the average size of pilomatrix carcinomas reported in the literature is 3.6-4.6 cm [3,4], the insidious nature of this disease can lead to large tumors precluding primary closure. However, invasive disease and the tendency for recurrence can limit the long term viability of complex reconstructions. Despite these challenges, literature on reconstruction following primary tumor resection is surprisingly sparse. Several authors including Sau et al. have reported the use of split-thickness skin graft, but the technique and outcomes are not well described [4]. Despite the paucity of literature, wide local excision combined with local reconstruction and skin grafting offer viable treatment and reconstructive options for this rare cutaneous malignancy with high recurrence rates.
References
- Lopansri S, Mihm MC Jr (1980) Pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe: a case report and review of literature. Cancer 45:2368-2373.
- Cornejo KM, Deng A (2013) Pilomatrix carcinoma: a case report and review of the literature. Am J Dermatopathol 35:389-394.
- Melancon JM, Tom WL, Lee RA, Jackson M, Jiang SI (2011) Management of pilomatrix carcinoma: a case report of successful treatment with Mohs micrographic surgery and review of the literature. DermatolSurg37:1798-1805.
- Sau P, Lupton GP, Graham JH (1993) Pilomatrix carcinoma. Cancer 71:2491-2498.
- Bremnes RM, Kvamme JM, Stalsberg H, Jacobsen EA (1999) Pilomatrix carcinoma withmultiple metastases: report of a case and review of the literature. Eur J Cancer 35:433-437.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences