Concurrent Skin Reduction in Nipple Sparing Mastectomy and Immediate Tissue Expander Reconstruction for Grade Three Ptosis Breasts
Hubert B Shih, Afaaf Shakir BS, Irene L Wapnir and Dung H Nguyen
DOI10.4172/2472-1905.100013
Hubert B Shih1*, Afaaf Shakir BS1, Irene L Wapnir2 and Dung H Nguyen1
Division of Plastic Surgery, Department of Surgery, USA
Department of Surgery / Section of Surgical Oncology, Stanford University School of Medicine, USA
- *Corresponding Author:
- Hubert B Shih
Division of Plastic Surgery
Department of Surgery, USA.
Tel: 4087714912
Fax: 6507256605
E-mail: hshih@stanford.edu
Received date: February 17, 2016; Accepted date: February 29, 2016; Published date: March 11, 2016
Citation: Shih HB, Afaaf Shakir BS, Wapnir IL, et al. Concurrent Skin Reduction in Nipple Sparing Mastectomy and Immediate Tissue Expander Reconstruction for Grade Three Ptosis Breasts. J Aesthet Reconstr Surg. 2016, 2:4. doi: 10.4172/2472-1905.100013
Keywords
Immediate breast reconstruction; Ptosis; Nipple sparing mastectomy; Tissue expander; Skin reduction
Introduction
The nipple-sparing mastectomy (NSM) is a significant advancement in the care of the breast cancer patient, allowing the potential for an excellent cosmetic result while maintaining oncologic benefits [1]. Indeed, preserving the nipple areolar complex (NAC) is shown to have increased patient satisfaction after breast reconstruction [1].
However, not all women are candidates for the operation. From an oncologic perspective, NSM is limited to women with small, early stage peripheral cancers [1,2]. From a plastic surgery perspective, the NSM is typically offered to women with small breasts and minimal ptosis. The large breasted woman with significant ptosis faces risks with this operation, including NAC and skin necrosis due to poor perfusion to the long mastectomy skin flaps, NAC malposition, and poor contour and profile of the breast due to redundant skin.
Several reports describe efforts to minimize the risks of skin necrosis and cosmetic deformity in such large ptotic breasts. One of the first published was by Woods who described concurrent NSM and mastopexy [1]. Although these approaches have improved the cosmetic results, NAC and mastectomy skin flap necrosis continue to be a problem. For example, Rivolin described a one-stage procedure with NSM and a peri-areolar pexy for the moderately ptotic breast. The series of 22 patients suffered an 18% NAC necrosis rate [1]. Rusby utilized a Wise pattern skinreducing NSM and performed de-epithelization around the NAC and subsequent in-folding of the dermis to aid in perfusion. Of the 17 breast reconstructions, one NAC was sacrificed due to poor perfusion, and post operatively there was one case of NAC necrosis [1]. Folli described a similar approach, but instead maintained the NAC on a longitudinally oriented dermal flap. In this series of 13 procedures, there were nine cases of NAC epidermolysis, though all healed uneventfully [1]. More recently, Spear reported success with a staged approach. Patients first underwent mastopexy or breast reduction, then at a second stage proceeded to have NSM and tissue expander reconstruction. In the series of 24 breasts, two breasts had skin flap necrosis with one of these, requiring expander explantation, while three breasts suffered from partial NAC necrosis.
We report a technique used in two patients with grade three ptosis whereby an initial NAC devascularization procedure is performed, followed four weeks later with NSM and skin reduction using either a Wise or peri-areolar pattern de-epithelialization and infolding technique, and placement of a tissue expander. With this method, there have been no complications with NAC or skin viability.
Method
Patients are deemed acceptable candidates for NSM from an oncologic perspective [3,4] and are evaluated by the plastic surgeon. The reconstructive options are discussed, including both autologous and implant based options. Two patients with grade three ptosis who desired NSM and immediate reconstruction with tissue expanders were identified as candidates of staged reconstruction starting with NAC devascularization.
Devascularization was performed using a previously reported technique [5-10] whereby the NAC along with several centimeters of surrounding skin is elevated in the mastectomy plane. Sub-areolar biopsy is also done at the same time. During devascularization, adequate nipple perfusion is confirmed intraoperatively using indocyanine green [11].
Case 1
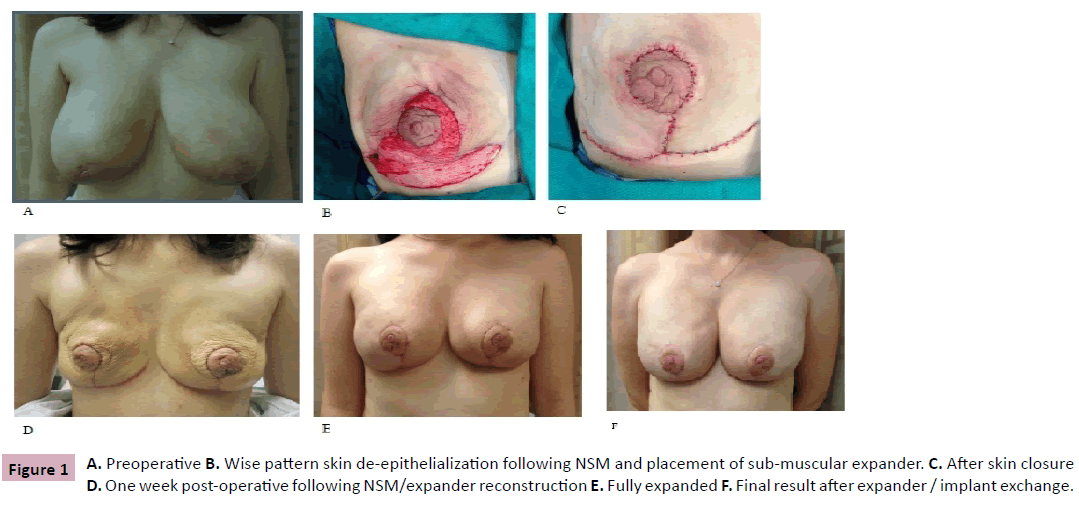
A 32 year-old female with BRCA2 genetic mutation with a diagnosis of infiltrating ductal carcinoma of the right breast, T2N0M0. Preoperatively, she underwent neoadjuvant chemotherapy with a complete clinical response. No post-mastectomy radiation was indicated. She had large pendulous breasts (Measurements: (B/L) S-N 29 cm, (B/L) N-IMF 10.5 cm) with large areolas and grade three ptosis (Figure 1A). She was a nonsmoker with no other medical problems.
She underwent bilateral devascularization of the NAC’s and sub-areolar biopsies, along with right breast lumpectomy and sentinel node biopsy by the breast surgeon. The procedure was approached using the lateral limbs of the eventual Wise reduction pattern. Skin flaps in the avascular plane were elevated in a circumferential manner using a number 15 scalpel to approximately seven centimeters beyond the edge of the areola. Hemostasis was achieved and the breast incisions were closed using interrupted 3-0 Vicryl dermal sutures and subcuticular 4-0 PDS sutures. No drains were placed. Incisions were covered with Steri-Strips, gauze, and Tegaderm dressings. The patient was instructed to apply antibiotic ointment to the NAC’s twice a day and to wear a sports bra to minimize seroma formation. There was mild epidermolysis of the NAC that healed within seven to ten days post-operative.
Four weeks later, bilateral NSM and reconstruction were performed. The mastectomies were performed by the breast oncologic surgeon via the lateral limb of the Wise pattern skin incision. The mastectomy skin flap was 4 mm thick. A submuscular 570 ml Sientra (Sientra, Inc., Santa Barbara, CA) double chamber tissue expander was placed, with AlloDerm (LifeCell Corp., Bridgewater, NJ) used to cover the inferior pole of the tissue expander. With the skin flaps draped over the properly positioned expander, the remainder of the Wise pattern incision was made thru the epidermis. The Wise pattern was then de-epithelialized using an iris scissors (Figure 1B). The exposed dermis was infolded and then the skin was closed with 3-0 Vicryl dermal sutures and interrupted 4-0 clear nylon for the skin (Figure 1C). The expander was not inflated to avoid compression of the in-folded dermal plexus. A #15 Blake subcutaneous drain had been placed. The procedure was repeated on the contralateral breast. There were no problems with skin flap or NAC survival and healing was uneventful (Figure 1D). Post-operatively, the patient was placed in a breast binder with light compression and kept warm with a Bair hugger and woolen post-operative pads (Lanacare, Vilnius, Lithuania) overnight.
Two and a half months later, after expansion to 500 and 450 ml in the right and left breasts, respectively (Figure 1E), the expanders were removed, capsulotomies were performed and Allergan (Allergan plc, Dublin, Ireland) 410 Style FF 655 ml implants were placed in both breasts. In addition, minor revision to further reduce the areola and 120 ml of fat grafting to the superior pole of both breasts were also done. The patient again healed uneventfully, with post-operative results shown in Figure 1F.
Case 2
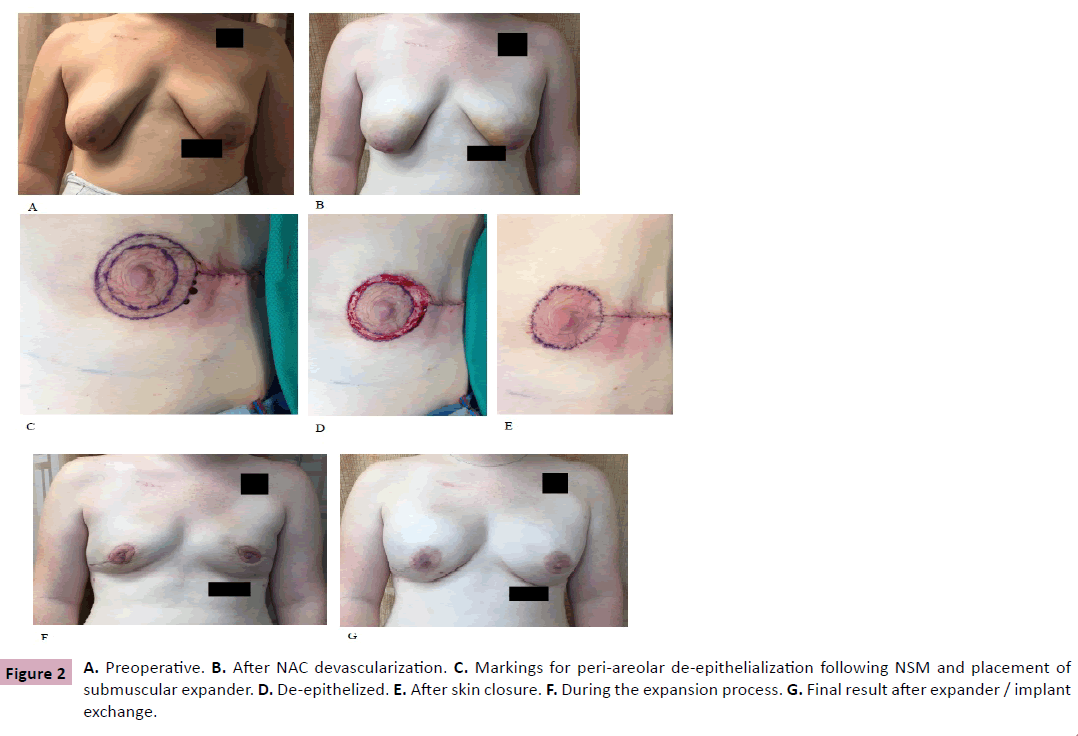
A 22 year-old female with a BRCA1 mutation who desired prophylactic bilateral mastectomy and tissue expander reconstruction. She had large tuberous breasts (Measurements S-N (R) 29.5 cm, (L) 28.5 cm; N-IMF (R) 11 cm, (L) 10 cm with grade three ptosis (Figure 2A). She underwent bilateral devascularization of the NAC along with sub-areolar biopsies. The procedure was approached using a lateral radial incision. Skin flaps in the avascular plane were elevated in a circumferential manner approximately 3 cm beyond the edge of the areola (Figure 2B). Otherwise the surgical technique and post-operative care regimen was similar to Case 1.
Four weeks later, bilateral NSM and reconstruction were performed. The mastectomies were performed by the breast oncologic surgeon via the prior lateral radial incisions. The mastectomy skin flap was 6 mm thick. A submuscular Allergan 133MX-15-T 700 ml tissue expander was then placed, with AlloDerm used to cover the inferior pole of the tissue expander. With the skin flaps draped over the properly positioned expander, a peri-areolar skin pattern was incised through the epidermis and the area was deepithelialized. The exposed dermis was infolded and then the skin was closed with 3-0 Vicryl dermal sutures and interrupted 4-0 clear nylon for the skin (Figures 2C-2E). The expander was not inflated. A #15 Blake subcutaneous drain had been placed. The procedure was repeated on the right breast. There were no problems with skin flap or NAC survival and healing was uneventful (Figure 2F). Patient received the same post-operative care and instructions as the previous patient. NAC’s and mastectomy skin healed well without peeling / epidermolysis.
Four months later, after expansion to 700 ml in both breasts, the expanders were removed, capsulotomies performed, and Allergan Inspira Implants 800 ml were placed in both breasts. In addition, 80 ml of fat grafting was injected to the superior pole of both breasts. The patient again healed uneventfully, with postoperative results shown in Figure 2G.
Results
Two patients with grade three ptosis underwent bilateral NAC devascularization followed by NSM and immediate tissue expander breast reconstruction with skin reduction in either a Wise pattern or peri-areolar pattern. The mastectomy was prophylactic in three breasts and therapeutic in one breast. Both patients were otherwise healthy nonsmokers. Acellular dermal matrix was used in all cases. In both patients, the time from NAC devascularization to NSM was four weeks. In the cancer patient, time from NSM and expander placement to final expander / implant exchange was two-and-a-half months, while for the non-cancer patient it was four months. There was no skin flap or NAC necrosis, infection, wound healing problems, implant malfunction, nor any other complications. The skin reduction allowed for a nice cosmetic result including a good NAC position and breast contour.
Discussion and Conclusion
The grade three ptosis and large breasted patient has long been a reconstructive challenge. To begin with, the redundant skin poses difficulties with NAC positioning and achieving a pleasing breast contour and profile. Furthermore, despite the proven benefits of NSM, historically such patients have not been candidates for NSM due to high rates of skin and nipple necrosis and wound healing complications. There have been several techniques described in the literature to address this with variable results. We believe our staged technique of starting with NAC devascularization, followed by NSM and immediate tissue expander breast reconstruction with skin reduction offers a consistent and reliable solution.
Our approach offers several advantages. The NAC devascularization takes advantage of the well-established concept of surgical delay to allow adaptation in the vasculature to help endure the larger insult of subsequent NSM and expander placement. At the time of the NAC devascularization, sub-areolar biopsy can be performed and analyzed via permanent sections, which could potentially change the oncologic and reconstructive plan. Furthermore, in some published techniques relying on a staged approached, there is the potential for delay of cancer treatment. In our approach, the initial NAC devascularization allows the oncologic surgeon an opportunity to perform a lumpectomy to remove the tumor before the final NSM to avoid any delay of oncologic treatment. Our skin reduction technique is tailored to the breast to allow for an appropriate NAC position and breast contour, while preserving NAC perfusion. Careful skin de-epithelization is required to maintain a dermal pedicle for the NAC. The expander is not filled at the time of placement in order to avoid compressing or kinking the dermal plexus of the in-folded skin. Close cooperation with the oncologic surgeon is necessary to ensure that the mastectomy skin flaps are of appropriate thickness.
References
- Chen CM, Disa JJ, Sacchini V, Pusic AL, Mehrara BJ, et al. (2009) Nipple-sparing mastectomy and immediate tissue expander/implant breast reconstruction. PlastReconstrSurg 124: 1772-1780.
- Wellisch DK, Schain WS, Noone RB, Little JW III (1987) The psychological contribution of nipple addition in breast reconstruction. PlastReconstrSurg 80:699-704.
- Krajewski AC, Boughey JC, Degnim AC, Jakub JW, Jacobson SR, et al. (2015) Expanded Indications and Improved Outcomes for Nipple-Sparing Mastectomy Over Time. Ann SurgOncol 22:3317-3323.
- Spear SL, Hannan CM, Willey SC, Cocilovo C (2009) Nipple-sparing mastectomy. PlastReconstrSurg 123:1665-1673.
- Woods JE (1987) Detailed technique of subcutaneous mastectomy with and without mastopexy. Ann PlastSurg 18:51-61.
- Rivolin A, Kubatzki F, Marocco F, Martincich L, Renditore S, et al. (2011) Nipple-areola complex sparing mastectomy with periareolarpexy for breast cancer patients with moderately ptotic breasts. J PlastReconstrAesthetSurg65:296-303.
- Rusby JE, Gui GP (2010) Nipple-sparing mastectomy in women with large or ptotic breasts. J PlastReconstrAesthetSurg 63: e754-e755.
- Folli S, Mingozzi M, Curcio A, Buggi F, Rossi C (2015) Nipple-sparing mastectomy: an alternative technique for large ptotic breasts. J Am CollSurg 220:e65-e69.
- Spear SL, Rottman SJ, Seiboth LA, Hannan CM (2012) Breast Reconstruction Using a Staged Nipple-Sparing Mastectomy following Mastopexy or Reduction. PlastReconstrSurg 129: 572.
- Jensen JA, Lin JH, Kapoor N, Giuliano, AE (2012) Surgical Delay of the Nipple–Areolar Complex: A Powerful Technique to Maximize Nipple Viability Following Nipple-Sparing Mastectomy. Ann SurgOncol 19:3171-3176.
- Dua MM, Bertoni DM, Nguyen D, Meyer S, Gurtner GC, et al. (2015) Using intraoperative laser angiography to safeguard nipple perfusion in nipple-sparing mastectomies. Gland Surg 4:497-505.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences