A Systematic Review of Dexamethasone, Hydrocortisone, and Triamcinolone in the Management of Carpal Tunnel Syndrome
Xi Ming Zhu1,2*, Eyal Ben-David1,2, Peter MacNeal2 and Jamil Moledina2
1Department of Medicine, St. George’s Hospital, University of London, UK
2St. George’s University Hospitals, NHS Foundation Trust, UK
- *Corresponding Author:
- Dr. Xi Ming Zhu, MSc, MBBS
Department of Medicine
St. George’s University Hospitals NHS Foudnation Trust
Blackshaw Rd, London, UK
E-mail: ming.x.zhu.89@gmail.com
Received Date: July 22, 2021; Accepted Date: August 10, 2021; Published Date: August 17, 2021
Citation: Zhu XM, Ben-David E, MacNeal P, Moledina J (2021) A Systematic Review of Dexamethasone, Hydrocortisone, and Triamcinolone in the Management of Carpal Tunnel Syndrome. J Aesthet Reconstr Surg Vol.7 No.5:38.
Abstract
Background: Carpal tunnel syndrome is a common cause of morbidity amongst adults. It is amenable to multiple therapeutic interventions, ranging from splinting to surgical decompression. The decision as to which steroid to use for local injection in carpal tunnel syndrome remains the subject of clinical equipoise. This systematic review provides an up-to-date summary of evidence for steroid injection in carpal tunnel syndrome.
Methods: A comprehensive search of Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) was performed covering from January 1st 1946 to October 12th 2020.
Results: Triamcinolone demonstrated efficacy in reducing distal motor latency and pain on VAS. Triamcinolone and dexamethasone demonstrated a significant reduction in distal sensory latency. There was insufficient data available to compare the three steroids to one another.
Conclusion: We demonstrate efficacy of triamcinolone and highlight a lack of evidence to make conclusive statements about dexamethasone and hydrocortisone.
Keywords
Carpal tunnel; Steroids; Plastic surgery
Introduction
Carpal tunnel syndrome (CTS) refers to the symptoms that arise owing to compression of the median nerve as it passes under the flexor retinaculum at the wrist. This compressive neuropathy results in pain, paraesthesia and weakness in the muscles of the hand supplied by the median nerve. CTS is a common disorder that affects 1–5 percent of adults in developed nations [1-3]. It is the most common peripheral nerve entrapment syndrome in adults, representing significant morbidity and cause of reduced productivity [4].
Treatment for carpal tunnel may be broadly classified as surgical and non-surgical. Non-surgical modalities include splinting, physiotherapy, and injection of glucocorticoids. Non-surgical treatments are generally used in mild to moderate CTS, whereas surgical decompression is offered in severe or treatment-resistant manifestations. A 2007 Cochrane systematic review demonstrated glucocorticoid injections provide greater symptomatic relief than placebo; however, the symptomatic relief was transient [5].
This widely used, minimally invasive technique provides rapid symptom relief and may be repeated if symptoms recur.
Different glucocorticoid injections are used for the treatment of CTS. Owing to a limited number of studies directly comparing glucocorticoids, with significant variation in study design, quality and population characteristics, it is unknown which steroid is the safest and most efficacious. This systematic review provides an up-to-date review of the literature comparing the outcomes from different glucocorticoid injections.
Research Methodology
This study was conducted according to the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions [6], and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO). CRD42020202792.
Search and screening strategy
A comprehensive search of Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) was performed covering from January 1st 1946 to October 12th 2020. The search strategy can be found in Table 1. The title, abstract, and full text screening was performed by two reviewers (XMZ and EBD) independently and in duplicate using piloted screening forms. Disagreements during screening moved onto the next stage for further in-depth review. Discrepancies between reviewers were discussed with the senior author (PM) and the principal investigator (JM).
| Search number | Search term | Ovid Medline and Embase | CENTRAL |
|---|---|---|---|
| 1 | Carpal tunnel | 29243 | 1527 |
| 2 | Carpal tunnel syndrome | 27525 | 1458 |
| 3 | Dexamethasone | 239312 | 11887 |
| 4 | Hydrocortisone | 215634 | 9484 |
| 5 | Triamcinolone | 41979 | 3245 |
| 6 | 1 or 2 | 29243 | 1568 |
| 7 | 3 or 4 or 5 | 462019 | 23596 |
| 8 | 6 and 7 | 457 | 89 |
| 9 | Remove duplicates from 8 | 375 | - |
Table 1 Search strategy and number of results on Ovid Medline, Embase, and CENTRAL.
Inclusion and exclusion criteria
Included studies were primary studies in the English language with usable data looking at adult patients with CTS involving one or both hands in the absence of peripheral neurological conditions, treated using primary corticosteroid injection with dexamethasone, hydrocortisone, or triamcinolone. Studies that were in another language, non-human subjects, reviews, case reports, commentaries, editorials, and conference abstracts were excluded. In studies that used the same population, the study with the larger patient pool was used. For studies that looked at different doses of the same steroid, the population group receiving the higher dosage was used.
Data extraction
A predefined form was used by each independent reviewer to extract data from the selected studies. This included title, authorship, number of patients for each study, mean age, and follow-up period. Outcome measures for pre and post intervention periods were extracted. These included clinical severity scores: DASH, PRWE, GSS, pain, and grip strength, and neurophysiology measures including mean motor latency and mean sensory latency.
Statistical analysis
Kappa score was used to assess agreement between the reviewers during the study screening. Based on the guidelines of Landis and Koch, a κ of 0 to 0.2 represents slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, and 0.61–0.80 substantial agreement [7]. A value above 0.80 is considered near perfect agreement. The paired student t-test was used to compare pre- and post-injection outcomes for each individual steroid, and outcomes between steroids were compared using the unpaired student t-test. P < 0.05 was considered to be statistically significant
Results
Study selection
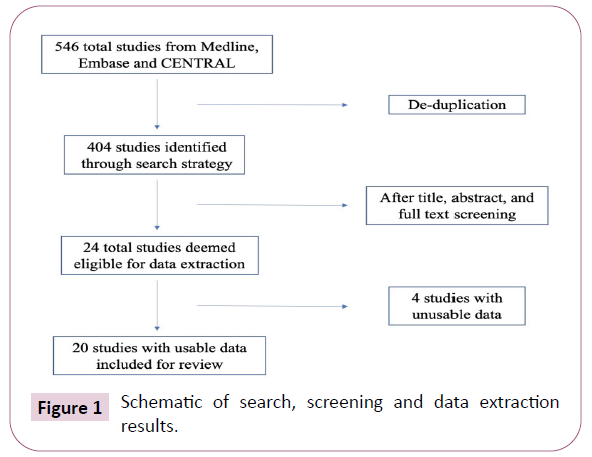
The initial search of online databases yielded 457 titles from Ovid Medline and Ovid Embase and 89 titles from CENTRAL. After deduplication, this resulted in a total of 404 studies that underwent screening. Using pre-determined criteria to screen titles, abstracts, and full texts, a total of 24 eligible studies were used in this systematic review. Six of these studies did not have any relevant or usable data, and were subsequently excluded, for a total of 20 studies [8-25]. A summary of the studies and treatment modalities examined can be found in Table 2. Literature search and screening results can be found in Figure 1.
| Study | Treatment, number of patients and mean age | Length of follow-up | Study design | ||
|---|---|---|---|---|---|
| D | H | T | |||
| Hsu et al. | - | - | 28 (57.1) | 12 weeks | RCT – double blinded |
| Karimzadeh et al. | - | - | 20 (54.8) | 12 weeks | RCT – double blinded |
| Wu et al. | - | - | 27 (54.3) | 6 months | RCT – double blinded |
| Dilokhuttakarn et al | 30 (48.7) | - | 30 (49.3) | 8 weeks | RCT – double blinded |
| Raeissadat et al. | - | - | 41 (51) | 6 months | RCT |
| Bahrami et al. | - | - | 32 (51.7) | 10 weeks | RCT – double blinded |
| Lee JY et al. | - | - | 15 (50.3) | 12 weeks | RCT |
| Soltani et al. | - | 17 (46.7) | - | 8 weeks | RCT |
| Seok et al. | - | - | 16 (49.7) | 3 months | RCT |
| Deniz et al. | 17 (46) | - | 8 weeks | Prospective cohort | |
| Karadas et al. | - | - | 20 (46.4) | 6 months | RCT |
| Karadas et al. | - | - | 34 (48) | 6 months | RCT – double blinded |
| Moghtaderi et al. | 20 (30) | - | - | 3 weeks | Prospective cohort |
| Lee JH et al. | - | - | 14 (52) | 8 weeks | Prospective cohort |
| Dewi et al. | - | - | 25 (53.6) | 4 weeks | RCT |
| O’Gradaigh | - | 16 | 18 | 6 weeks | RCT |
| Giannini et al. | - | - | 31 (55.2) | 6 months | Prospective cohort |
| Rayegani et al. | - | - | 23 (54) | 10 weeks | RCT |
Table 2 Summary of studies included in this review.
Agreement on study inclusion between reviewers for title was moderate (κ: 0.593 SE: 0.070), for abstract was near perfect (κ: 0.837 SE: 0.078), and for full text was near perfect (κ: 0.902 SE: 0.096).
Distal motor latency
Two studies with 37 patients reported distal motor latency (DML) at latest follow-up for dexamethasone, which had a mean value of 4.35 ms [17,20]. Two studies with 33 patients reported DML at latest follow-up for hydrocortisone, which had a mean value of 4.2 ms [15,23]. Finally, 14 studies with 344 patients receiving triamcinolone reported a mean DML value of 4.57 ms [8-10,12- 16,18,19,21-25] (Table 3).
| Study | Mean DML (ms) | Mean DSL (ms) | VAS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D | H | T | D | H | T | D | H | T | |
| Hsu et al. | - | - | 4.89 | - | - | - | - | - | 3 |
| Karimzadeh et al. | - | - | 4 | - | - | 4 | - | - | 3.7 |
| Wu et al. | - | - | 5 | - | - | - | - | - | 4.5 |
| Dilokhuttakarn et al | - | - | - | - | - | - | - | - | - |
| Raeissadat et al. | - | - | 3.9 | - | - | 3.5 | - | - | 2.7 |
| Bahrami et al. | - | - | 4.04 | - | - | 3.94 | - | - | 2.3 |
| Lee JY et al. | - | - | 4.68 | - | - | 4.08 | - | - | - |
| Soltani et al. | - | 4 | - | - | 3.8 | - | - | 2.5 | - |
| Seok et al. | - | - | 4.37 | - | - | 3.28 | - | - | 3.31 |
| Deniz et al. | 4 | - | - | 3.8 | - | - | - | - | - |
| Karadas et al. | - | - | 4.76 | - | - | - | - | - | 4.76 |
| Karadas et al. | - | - | 4.9 | - | - | - | - | - | 4.9 |
| Moghtaderi et al. | 4.7 | - | - | 4.2 | - | - | 4.3 | - | - |
| Lee JH et al. | - | - | 4.8 | - | - | 4.7 | - | - | 1.4 |
| Dewi et al. | - | - | 5.42 | - | - | 3.39 | - | - | - |
| O’Gradaigh | - | 4.4 | - | - | - | - | - | - | - |
| Giannini et al. | - | - | 4.3 | - | - | - | - | - | - |
| Rayegani et al. | - | - | 4.32 | - | - | 4.12 | - | - | 3.04 |
Table 3 Summary of mean DML, DSL, and VAS for included studies at latest follow-up.
A comparison of pre and post injection mean DMLs reveals that patients receiving triamcinolone had a statistically significant reduction in DML at the latest follow-up, mean difference (MD) -0.56 (95% CI: -0.33:-0.78, p=0.0001). No statistically significant difference was found for dexamethasone or hydrocortisone.
There was no statistically significant difference between the three different steroids (Table 4).
| Steroid Used | Distal motor latency (ms) | Paired t-test result | Distal sensory latency (ms) | Paired t-test result | Pain on VAS | Paired t-test result | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||||
| Dexamethasone | 4.68 | 4.35 | MD: 0.33 | 4.29 | 4 | MD: 0.29 | 8.7 | 4.3 | - |
| CI: -1.32 – 1.98 | CI: 0.163 – 0.417 | ||||||||
| p = 0.239 | p = 0.022 | ||||||||
| Hydrocortisone | 4.5 | 4.2 | MD: 0.30 | 4.3 | 3.8 | - | 6 | 2.5 | - |
| CI: -2.24 – 2.84 | |||||||||
| p = 0.374 | |||||||||
| Triamcinolone | 5.13 | 4.57 | MD: 0.56 | 4.43 | 3.88 | MD: 0.55 | 5.84 | 3.37 | MD: 2.48 |
| CI: 0.33 – 0.78 | CI: 0.359 – 0.75 | CI: 1.64. – 3.31 | |||||||
| p = 0.0001 | p = 0.002 | p = 0.0001 | |||||||
Table 4 Mean DML, DSL, and VAS pre and post injection, and comparison of pre and post injection using paired t-test.
Distal Sensory Latency
Two studies with 37 patients reported distal sensory latency (DSL) at the latest follow-up for dexamethasone, which had a mean value of 4.00 ms [17,20]. Only one study reported DSL for hydrocortisone [15]. Eight studies with 186 patients reported DSL for patients receiving triamcinolone and had a mean value of 3.88ms at the latest follow-up [9,12-14,16,21,22,25].
A comparison of pre and post injection mean DSLs found that patients receiving triamcinolone experienced a statistically significant reduction in DSL, MD: -0.55 (95% CI -0.36:-0.75, p=0.002). Patients receiving dexamethasone also experienced a significant reduction in DSL post injection, MD: -0.29 (95% CI -0.16:-0.42, p=0.022).
There was no statistically significant difference between dexamethasone and triamcinolone, MD: 0.123 (95% CI -0.625:0.872, p=0.718). No analysis on hydrocortisone could be done owing to insufficient data (Table 5).
| Variables | Distal motor latency | Distal sensory latency | Pain on VAS |
|---|---|---|---|
| D vs. H | MD: 0.150 | - | - |
| CI: -1.58 – 1.88 | |||
| p = 0.7455 | |||
| D vs. T | MD: -0.22 | MD: 0.123 | - |
| CI: -0.932 – 0.492 | CI: -0.625 – 0.872 | ||
| p = 0.518 | p = 0.718 | ||
| H vs. T | MD: -0.37 | - | - |
| CI: -1.059 – 0.319 | |||
| p = 0.269 |
Table 5 Comparison of post injection DML, DSL and VAS of dexamethasone, hydrocortisone, and triamcinolone against each other using the unpaired t-test.
An improvement in DSL post-procedure was observed with both dexamethasone and triamcinolone, with mean reductions of 0.29 ms and 0.55 ms, respectively. This statistically significant improvement was determined in a cohort of only 37 patients receiving dexamethasone. The studies for hydrocortisone were underpowered to demonstrate significance.
Pain
Only one study reported pain as measured by the visual analogue scale (VAS) for dexamethasone and hydrocortisone in 20 and 17 patients, respectively [15,20]. Ten studies with 255 patients reported VAS for patients receiving triamcinolone. No analysis between different steroid groups could be done owing to insufficient data for dexamethasone and hydrocortisone groups [8-10,12,13,16,18,19,21,25]. Patients receiving triamcinolone showed a significant reduction in pain post injection, MD: -2.48 (95% CI -1.64:-3.31, p=0.0001).
Discussion
We provide an up-to-date summary of studies comparing different steroid formulations for treatment of CTS. Using 20 available papers, we have directly compared three corticosteroids: dexamethasone, triamcinolone, and hydrocortisone with regards to specific outcomes. Grip strength and functional scores were not analysed owing to insufficient data.
Of the three steroid injections reviewed, only triamcinolone demonstrated efficacy at reducing DML. This was with an average reduction of 0.56 ms in the 344 patients observed. It is important to note that only 37 and 33 patients for dexamethasone and hydrocortisone cohorts respectively were available for analysis. To accurately ascertain whether hydrocortisone and dexamethasone truly reduce DML, further studies need to be performed.
The only corticosteroid with sufficient data to demonstrate an improvement in post-procedural pain was triamcinolone, with a reduction of 2.48 on the 10-point VAS. This clinically significant reduction in pain establishes triamcinolone as an excellent therapeutic option in the management of CTS. Unfortunately, there is insufficient data to make inferences of the effectiveness of hydrocortisone and dexamethasone at improving CTS pain. Larger studies of hydrocortisone and dexamethasone reporting pain on a 10-point VAS need to be performed.
While minor adverse effects including transient pain were described [8,12], none of the studies reported significant adverse outcomes related to glucocorticoid injection. Within wider literature, major complications such as intraneural injection of glucocorticoids have been reported [26]; however, there is currently insufficient evidence to comment on the comparative safety of the different glucocorticoids for CTS.
Conclusion
Patients receiving triamcinolone can expect an improvement in DMS and DSL and pain. Data was lacking to report on dexamethasone and hydrocortisone. Owing to paucity of comparative studies, it was not possible to compare dexamethasone, hydrocortisone and triamcinolone to one another.
Disclosure of Potential Conflicts of Interest and Funding
The authors declare no conflicts of interest to report in the production of this study. This research received no specific grant from any funding agency in the public, commercial, or not-forprofit sectors.
Statement of Human and Animal Rights
Human studies included in this article contain no identifiable information. This article does not contain any studies with animal subjects.
Statement of Informed Consent
No patient identifiable information is present in this systematic review and as such, no consent was required for its production.
References
- Pourmemari MH, Heliövaara M, Viikari-Juntura E, Shiri R (2018) Carpal tunnel release: Lifetime prevalence, annual incidence, and risk factors. Muscle Nerve 58: 497-502.
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, et al. (1999) Prevalence of carpal tunnel syndrome in a general population. J Amer Med Assoc 282: 153.
- De Krom MCTFM, Kester ADM, Knipschild PG, Spaans F (1990) Risk factors for carpal tunnel syndrome. Am J Epidemiol 132: 1102-1110.
- Caliandro P, La Torre G, Aprile I, Pazzaglia C, Commodari I, et al. (2006) Distribution of paresthesias in Carpal Tunnel Syndrome reflects the degree of nerve damage at wrist. Clin Neurophysiol 117: 228-231.
- Marshall S, Tardif G, Ashworth N (2007) Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberatiet A, et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. Br Med J 349: g7647.
- Landis JR, Koch GG (1977) The Measurement of Observer Agreement for Categorical Data. Int Biometric Soc 33: 159.
- Hsu PC, Liao KK, Lin KP, Chiu JW, Wu PY, et al. (2020) Comparison of Corticosteroid Injection Dosages in Mild to Moderate Idiopathic Carpal Tunnel Syndrome: A Randomized Controlled Trial. Arch Phys Med Rehabil 101: 1857-1864.
- Karimzadeh A, Bagheri S, Raeissadat SA, Bagheri S, Rayeganiet SM, et al. ( 2019) The comparison of the effectiveness between different doses of local methylprednisolone injection versus triamcinolone in Carpal Tunnel Syndrome: A double-blind clinical trial. J Pain Res 12: 579-584.
- Wu YT, Ke MJ, Ho TY, Li TY, Shen YP, et al. (2018) Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann Neurol 84: 601-610.
- Dilokhuttakarn T, Lertnantapanya S, Vechmamontien S, Suwanchatchai C (2018) The efficacy of dexamethasone sodium phosphate compared to triamcinolone acetonide in the treatment of carpal tunnel syndrome: A randomized double-blind controlled trial. J Med Assoc Thailand.
- Raeissadat SA, Shahraeeni S, Sedighipour L, Vahdatpour B (2017) Randomized controlled trial of local progesterone vs corticosteroid injection for carpal tunnel syndrome. Acta Neurologica Scandinavica 136: 365-371.
- Bahrami MH, Shahraeeni S, Raeissadat SA (2015) Comparison between the effects of progesterone versus corticosteroid local injections in mild and moderate carpal tunnel syndrome: A randomized clinical trial. BMC Musculoskelet Disord p. 16.
- Lee JY, Park Y, Park KD, Lee JK, Lim OK (2014) Effectiveness of ultrasound-guided carpal tunnel injection using in-plane ulnar approach: A prospective, randomized, single-blinded study. Medicine 93: e350.
- Reza SZ, Mahsa A, Ahmad RS, Kamran G (2013) Low-level laser therapy versus local steroid injection in patients with idiopathic carpal tunnel syndrome: A single blind randomized comparative trial. Internet Journal of Medical Update – E-Journal.
- Seok H, Kim SH (2013) The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: A randomized controlled trial. Am J Physical Med Rehabilitation 92: 327-334.
- Deniz O, Aygül R, Kotan D, Özdemir G, Odabaş FO, et al. (2012) The eVect of local corticosteroid injection on F-wave conduction velocity and sympathetic skin response in carpal tunnel syndrome. Rheumatol Int 32: 1285-1290.
- Karadaş Ö, Tok F, Akarsu S, Tekin L, Balaban B (2012) Triamcinolone acetonide vs procaine hydrochloride injection in the management of carpal tunnel syndrome : Randomized placebo-controlled trial. J Rehabil Med 44: 601-604.
- Karadaş Ö, Tok F, Ulaş ÜH, Odabaşi Z (2011) The effectiveness of triamcinolone acetonide vs. procaine hydrochloride injection in the management of carpal tunnel syndrome: A double-blind randomized clinical trial. Am J Phy Med Rehabil 90: 287-292.
- Moghtaderi AR, Moghtaderi N, Loghmani A (2011) Evaluating the effectiveness of local dexamethasone injection in pregnant women with carpal tunnel syndrome. J Res Med Sci 16: 687-690.
- Lee JH, An JH, Lee SH, Hwang EY (2009) Effectiveness of steroid injection in treating patients with moderate and severe degree of carpal tunnel syndrome measured by clinical and electrodiagnostic assessment. Clin J Pain 25: 111-115.
- Dewi JI, Sadeli HA, Kurniani N, Gunadharma S (2009) A randomized study camparing oral versus injection triamcinolone in carpal tunnel syndrome. Neurol Asia 16: 247-249.
- O’Gradaigh D, Merry P (2000) Corticosteroid injection for the treatment of carpal tunnel syndrome. Ann Rheum Dis 918-919.
- Giannini F, Passero S, Cioni R, Paradiso C, Battistini N,et al. (1991) Electrophysiologic evaluation of local steroid injection in carpal tunnel syndrome. Arch Phys Med Rehabil 2: 227.
- Rayegani SM, Raeissadat SA, Ahmadi-Dastgerdi M, Bavaghar N, Rahimi-Dehgolan S (2019) Comparing the efficacy of local triamcinolone injection in carpal tunnel syndrome using three different approaches with or without ultrasound guidance. J Pain Res 12: 2951-2958.
- Kaile E, Bland JDP (2018) Safety of corticosteroid injection for carpal tunnel syndrome. J Hand Surg 43: 296-302.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences