Brazilian Butt Lift Procedures when the Renuvion APR System was used as an Adjunct Procedure: A Retrospective Chart Review.
Alexandre S.T. De Souza1*, Alexander Cole De Souza2
1Department of General Surgery, CG Cosmetic Surgery, Miami, United States
2University of Miami, Miami, United States
- *Corresponding Author:
- Alexandre S.T. De Souza
Department of General Surgery, CG Cosmetic Surgery, Miami, United States
E-mail: drdesouzabblposop@gmail.com
Received date: July 11, 2022, Manuscript No. IPARS-22-14109; Editor assigned date: July 13, 2022, PreQC No. IPARS-22-14109 (PQ); Reviewed date: July 24, 2022, QC No. IPARS-22-14109; Revised date: August 04, 2022, Manuscript No. IPARS-22-14109 (R); Published date: August 11, 2022, DOI: 10.36648/2472-1905.8.4.150
Citation: De Souza AST, De Souza AC (2022) Brazilian Butt Lift Procedures when the Renuvion APR System was used as an Adjunct Procedure: A Retrospective Chart Review. J Aesthet Reconstr Surg Vol.8 No.4:150.
Abstract
Background: The Brazilian Butt Lift (BBL) procedure has been in practice since the 1980’s and was introduced as a combination of liposuction and gluteal augmentation using fat transfer. Since its introduction, this procedure continues to evolve and incorporate other procedures and treatments to enhance the results. Among these procedures and treatments are liposuction of the abdomen and/or flanks, liposuction of the thighs, abdominoplasty, and heliumbased plasma RF device treatment. The ability to enhance the results of a BBL procedure by treating the surrounding body area with an alternative treatment could benefit the patients and the providers alike. This could bring the patients closer to achieving their goals of having plastic surgery and the provider could potentially optimize their care and have more consistent and positive outcomes.
Objective: To collate retrospective procedure data of Brazilian Butt Lift (BBL) procedures when the helium-based plasma RF device for soft tissue coagulation was used as an adjunct treatment.
Methods: A retrospective chart review was conducted (with a waiver of consent granted by the IRB) of patients aged ≥18 years who underwent treatment for a Brazilian Butt Lift (BBL) with a helium-based plasma RF device (Renuvion; Apyx Medical) utilized for soft tissue coagulation at one clinical site. Demographic data, procedure details, length of follow-up, and postoperative adverse events (AEs) and complications were recorded. Patient satisfaction questionnaires (PSQ) were also recorded for subjects consenting (via an IRB approved consent form) to study participation and obtained by telephone contact.
Results: Chart review identified 49 patients who underwent BBL procedures with the use of the helium-based plasma RF device as an adjunct treatment between March 4, 2021 and July 27, 2021. Of these patients, 31 had data regarding the helium-based plasma RF device treatment in conjunction with the BBL procedure. 29 consented to study participation and completed the PSQ survey. Only female subjects were enrolled in this study. Patients had an average age of 32 years (range 20-46 years) and an average BMI of 28.13 (range 21.6-35.4) at the time of treatment.
Most subjects (n=35) had a BBL procedure with Lipo 360 and an adjunct treatment on their abdomen and flanks utilizing the helium-based plasma RF device. In all the procedures, the helium-based plasma RF device was not utilized to treat the buttocks directly, but rather was used to smooth the areas between the back and the buttocks where the BBL procedure was, as well as in other areas of the body after liposuction. All areas treated with the helium-based plasma RF device were done so with treatment settings of 80% Power and 2.0-2.5 liters per minute (L/min) of Helium Flow. The only listed energy level (kJ) was 6.0kJ.
Conclusion: The purpose of this study was to evaluate safety and procedural information for Brazilian Butt Lift (BBL) procedures with the helium-based plasma RF device as an adjunct treatment. A chart review of 49 female patients was conducted with an additional Patient Satisfaction Questionnaire (PSQ) being completed by 29 patients. Based on the data, the helium- based plasma RF device settings were mostly 80% Power and 2.5 L/min Helium Flow Rate for various body areas treated. The most common areas treated were the abdomen and bilateral flanks. Patient satisfaction data showed that, out of those surveyed, most patients reported overall positive levels of satisfaction and noted positive improvements of their skin in the treated area(s). Furthermore, the data showed that this study is limited due to the retrospective design and data availability during chart review.
Introduction
In the 1980s, during the early use of injecting fat for the augmentation of various body areas, the Brazilian butt lift (BBL), or gluteal augmentation through fat transfer, was first introduced by Luiz S. Toledo [1]. Throughout the 1990’s he further developed his techniques on liposculpture for the face and body and began giving lectures in Brazil and abroad for the American Society for Aesthetic Plastic Surgery (ASAPS), the Lipoplasty Society of North America (LSNA), the International Society of Aesthetic Plastic Surgery (ISAPS), and the American Society of Plastic Surgeons (ASPS) [2]. Since then, gluteal augmentation has become one of the fastest-growing aesthetic procedures in the United States with the ASAPS reporting more than 20,000 procedures performed in 2016 alone [1].
The goal of a BBL procedure is to harvest fat from one or more body areas utilizing liposuction and then transferring the fat via injection to the gluteal area with specific attention made to the projection, contour, and height of the fat transfer [3]. In this study, the participating investigator introduces the early use of the helium-based plasma radiofrequency (RF) device (Renuvion®, Apyx™ Medical Corporation, Clearwater, FL) as an adjunct treatment to his BBL procedures. The subdermal application of energy using a helium-based plasma RF device has been shown to improve skin laxity. A helium-based plasma device (Renuvion®, Apyx™ Medical Corporation, Clear water, FL) has been cleared by the US Food and Drug Administration (FDA) for cutting, coagulation, and ablation of soft tissue (K191542). The participating investigator utilizes the helium-based plasma device for optimal tissue coagulation resulting in contraction outcomes to enhance the results of his BBL procedures.
The practice of applying heat to tissue using cauters has been prevalent for thousands of years and continuous improvements to these methods has led to the development what electrosurgery is today. Since the 1990s, radiofrequency (RF), laser, and plasma devices have been advocated to heat and cause collagen contraction [4-9]. Between 60°C and just below 100°C, protein denaturation leading to coagulation occurs, as well as cellular dehydration. As the temperature rises above 100°C, this intracellular water turns into steam and the tissue cells begin to vaporize. Understanding these heat effects of RF energy on cells and tissue can allow the predictable changes to be used to accomplish beneficial therapeutic results.
Out of these processes, protein denaturation is the process in which hydrothermal bonds between protein molecules are instantaneously broken and then quickly reformed as the tissue cools. This process leads to coagulation, where the cellular proteins are altered but not destroyed and form protein bonds. The resulting tissue effect of coagulation is useful for occluding blood vessels and causing hemostasis, as well predictable contraction of soft tissue. The coagulation and denaturation temperature of collagen is stated to be 66.8°C, although this can vary by tissue type [10]. Once denatured, collagen contracts as fibers shrink to one-third of their overall length [11]. Once tissue is heated to the appropriate temperature, protein denaturation, and collagen contraction occurs resulting in a reduction of volume and surface area of the heated tissue. Apyx Medical Corporation’s helium-based plasma technology passes helium gas over an energized electrode and generates the plasma passes from the electrode to the patient. Tissue is heated by passing a current through the resistance of the tissue, a process known as Joule heating.
Methods
A retrospective chart review of procedures performed by the Principal Investigator (PI) in which helium driven device was used for the coagulation of soft tissue as an adjunct procedure to a Brazilian Butt Lift (BBL) was conducted with a waiver of consent granted by Sterling IRB. The Electronic Medical Records (EMR) of a single medical clinic was reviewed to identify eligible subjects. Eligible subjects were males and females aged ≥18 years who underwent a BBL procedure in which a helium-based plasma RF device was used for soft tissue coagulation as an adjunct treatment.
Following the identification of eligible patient charts, study data were de-identified and documented. If available, the following de-identified information was collected: Patient identifier, date of birth, history & physical, consultation notes, reason for using a helium-based plasma RF device as an adjunct procedure, procedure date, procedure performed, treated body area(s), anesthesia method, treating surgeon, aspiration volume, complications (by treated area) during procedure, surgery time, technique, medications, area of fat transfer, aspirated amount of fat, amount of fat transferred, fat injection location, heliumbased plasma RF device treatment settings, helium-based plasma RF device treatment area(s), Adverse Events (AEs) reported post-operatively (if any), baseline imaging for up to 5 consented patients, follow-up imaging for up to 5 consented patients, and Patient Satisfaction Questionnaire for patients consenting to study participation.
Demographic data were collected, including sex, age, BMI, and weight. Pre-procedure details were collected, including preop diagnosis. Procedure details were collected, including procedures performed, area(s) of device application, total fat aspirated, and total fat transferred. Treatment settings were collected, including power as percentage and helium flow (L/ min). The amount of energy delivered (J) was listed and therefore was also collected for a couple (n=2) patients. Subject data generated after chart review was then summarized into 6 subcategories (Demographics, Pre-Op Diagnosis, Procedures Completed, Procedure Treatment Areas, Total Fat Aspirated and Transferred, and Patient Satisfaction).
Results
Chart review identified 49 patients who underwent BBL procedures with the use of the helium-based plasma RF device as an adjunct treatment between March 4, 2021 and July 27, 2021. Of these patients, 29 consented to study participation and completed the PSQ survey. Only female subjects were enrolled in this study. Patients had an average age of 32 years (range 20-46 years), an average BMI of 28.13 (range 21.6-35.4), and an average weight of 159.4 pounds (lbs.) (range 107-204 lbs.) at the time of treatment.
Chart review revealed that, of the listed pre-operative diagnoses, most patients (n=21) had a pre-operative diagnosis of lipodystrophy. Other patients had a pre-operative diagnosis of increased fat (n=1) or obesity (n=1). The remaining 26 patients did not have a pre-operative diagnosis listed. Most patients (n=35) underwent a BBL procedure with Lipo 360 and an adjunct treatment of their abdomen and flanks utilizing the heliumbased plasma RF device. Eight patients underwent the same set of procedures as the majority with the addition of a cell saver treatment, two with the addition of treating their thighs with Lipo 360, one with the additions of Lipo 360 to treat their chin and arms, one with the additions of treating their back and arms with the helium-based plasma RF device, and one with just the addition of treating their arms with the helium-based plasma RF device (Table 1).
| Procedure | n=# of Procedures Performed |
|---|---|
| BBL, Lipo 360, helium-based plasma RF device treatment-abdomen & flanks | n=35 (71%) |
| BBL, Lipo 360, helium-based plasma RF device treatment-abdomen & flanks, Cell Saver | n=8 (16%) |
| BBL, Lipo 360 + thighs, helium-based plasma RF device treatment-abdomen & flanks | n=2 (4%) |
| BBL, Lipo | n=1 (2%) |
| BBL, Lipo 360 + chin and arms, helium-based plasma RF device treatment-abdomen & flanks | n=1 (2%) |
| BBL, Lipo 360, helium-based plasma RF device treatment-abdomen, flanks, back, & arms | n=1 (2%) |
| BBL, Lipo 360, helium-based plasma RF device treatment-abdomen, flanks, & arms | n=1 (2%) |
Table 1: Procedures Completed
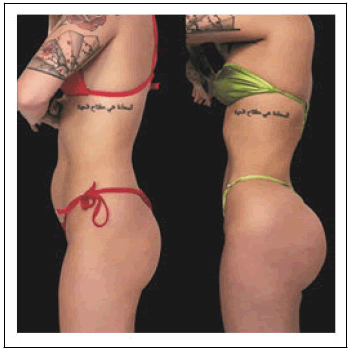
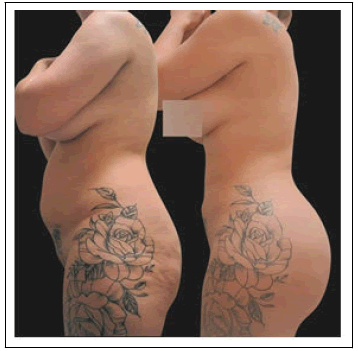
There was 1 patient whose chart review revealed they had undergone a BBL procedure with Liposuction, but the heliumbased plasma RF device was not reported to have been utilized. In all the procedures where the helium-based plasma RF device was utilized, it was not used to treat the buttocks directly, but rather was used to smooth the areas between the back and the buttocks where the BBL procedure was, as well as in other areas of the body after liposuction. (Figure 1).
Body areas treated with the helium-based plasma RF device were the abdomen, right flanks, left flank, right arm, left arm, and back. The majority of patients (n=29) had both their abdomen and bilateral flanks treated. All patients were treated with the helium-based plasma RF device at a power setting of 80%. The helium flow rate for all patients and all areas treated was either 2.0 or 2.5 varying on a case-by-case basis.The amount of energy applied during treatment was only listed for two procedure treatment areas but was 6.0kJ when treating the abdomen at 80% power with a helium flow rate of 2.5 L/ min (Figure 2 and Table 2).
| Treatment Area | Power (%) | Helium Flow Rate (L/min) | Energy (kJ) | Total |
|---|---|---|---|---|
| Abdomen | 80% | 2.5 | Not Listed | n=29 (94%) |
| Abdomen | 80% | 2 | Not Listed | n=1 (3%) |
| Abdomen | 80% | 2.5 | 6 | n=2 (7%) |
| Right Flank | 80% | 2.5 | 6 | n=2 (7%) |
| Right Flank | 80% | 2 | Not Listed | n=29 (94%) |
| Right Flank | 80% | 2 | Not Listed | n=1 (3%) |
| Left Flank | 80% | 2.5 | 6 | n=2 (7%) |
| Left Flank | 80% | 2.5 | Not Listed | n=29 (94%) |
| Left Flank | 80% | 2 | Not Listed | n=1 (3%) |
| Right Arm | 80% | 2.5 | Not Listed | n=1 (3%) |
| Left Arm | 80% | 2.5 | Not Listed | n=1 (3%) |
| Back | 80% | 2.5 | Not Listed | n=1 (3%) |
Table 2: Helium-based plasma RF device treatment areas and settings
The average amount (ml) of total fat aspirated documented was 3815ml. Most patients (n=28) had 4000ml fat aspirated during their procedure. Of the fat aspirated, the average amount (ml) of fat transferred was 1103ml with most patients (n=19) having 1200ml of fat transferred during their procedure. Of the aspirated fat, the average amount of fat transferred to each buttock (left and right) was approximately 370ml. Most patients (n=19) had 600 ml of the aspirated fat transferred to each buttock (left and right). The amount of fat transferred to each buttock was even across all patients.
Safety data collected from the chart review concluded that there were no Serious Adverse Events (SAEs) or unexpected adverse events reported while utilizing the helium-based plasma RF device in conjunction with the BBL procedure. There was no mention of any AEs post-surgery in the patient charts that were reviewed. An Adverse Event (AE) is any new problem, or the exacerbation of an existing problem, experienced by the subject while enrolled in the study whether considered device-related by the investigator or not whereas a Serious Adverse Event (SAE) is any new problem results in death, being life-threatening, hospitalization, disability or permanent damage, congenital anomaly/birth defect, requiring intervention to prevent permanent impairment or damage, or other event that does not fit the previously listed outcomes, but may jeopardize the patient and may require medical or surgical interventions to prevent the other outcomes. An unanticipated adverse event device effect is any serious adverse effect on health or safety, or any life-threatening problem, or death caused by, or associated with, a device; if that effect, problem, or death was not previously identified in nature, severity, or degree of incidence in the investigational plan, or application, or any other unanticipated serious problem associated with the device that relates to the rights, safety, or welfare of patients.
Of the 49 subjects, 29 were consented and completed the PSQ survey. When patients were asked about their satisfaction with their BBL procedure with helium-based plasma RF device treatment, 34% (n=10) were very satisfied, 52% (n=15) were satisfied, 7% (n=2) were slightly satisfied, 3% (n=1) were dissatisfied, and 3% (n=1) were very dissatisfied (Table 3A).
| How would you characterize your satisfaction with your BBL with helium-based plasma RF treatment? | |
| Very Satisfied | 34% (10 out of 29) |
| Satisfied | 52% (15 out of 29) |
| Slightly Satisfied | 7% (2 out of 29) |
| Neither Satisfied or Dissatisfied | 0% |
| Slightly Dissatisfied | 0% |
| Dissatisfied | 3% (1 out of 29) |
| Very Dissatisfied | 3% (1 out of 29) |
Table 3A: Patient Satisfaction Questionnaire (PSQ)
The majority of patients (82%, n=24) would recommend the BBL procedure with helium-based plasma RF device treatment and 17% (n=5) of patients would not (Table 3B).
| Would you recommend the BBL procedure with or without helium-based plasma RF treatment? | |
| With | 83% (24 out of 29) |
| Without | 17% (5 out of 29) |
Table 3B: PSQ (continued)
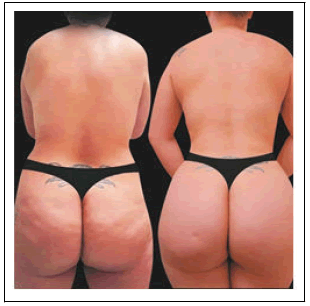
The same population, 83% (n=24) of patients would recommend the BBL procedure with helium-based plasma RF device treatment to their friends (Table 3C) and 79% (n=23) would consider having the BBL procedure with helium-based plasma RF device treatment performed again (Table 3D and Figure 2).
| Would you recommend the BBL procedure with helium-based plasma RF treatment to your friends? | |
| Yes | 83% (24 out of 29) |
| No | 17% (5 out of 29) |
Table 3C: PSQ (continued)
| Would you consider having the BBL procedure performed again with helium-based plasma RF treatment? | |
| Yes | 79% (23 out of 29) |
| No | 21% (6 out of 29) |
Table 3D: PSQ (continued)
When asked to rate their overall satisfaction on a scale of 1 to 10 (1=the worst, 10=the best), 93% of patients were satisfied with the procedure, listing a score of 6 or higher. Of those satisfied patients, 34% (n=10) chose 8 and 28% (n=8) chose 10 (Table 3E).
| On a scale of 1 to 10 (1 is the worst, 10 is the best), what is your overall satisfaction with your BBL procedure with helium- based plasma RF treatment? | |
| 1 (Worst) | 3% (1 out of 29) |
| 2 | 3% (1 out of 29 |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| 6 | 3% (1 out of 29) |
| 7 | 21% (6 out of 29) |
| 8 | 34% (10 out of 29) |
| 9 | 7% (2 out of 29) |
| 10 (Best) | 28% (8 out of 29) |
Table 3E: PSQ (continued)
When asked about the post-operative changes patients had seen in the treated area(s), they listed an improvement in skin tightness (n=27), skin feels better (n=22), and skin appears tighter (n=27) (Table 3F).
| Which, if any, changes do you see in the area(s) treated? | Yes | No | Total |
|---|---|---|---|
| Skin Tightness Improvement | 93% (27 out of 29) | 2 | 29 |
| Skin Feels Better | 76% (22 out of 29) | 7 | 29 |
| Skin Appears Tighter | 93% (27 out of 29) | 2 | 29 |
| Other-Fibrosis | 3% (1 out of 29) | n/a | 1 |
| None | 0% | 0 | 0 |
Table 3F: PSQ (continued)
When asked about their social and emotional wellbeing, most patients reported improvement in their social life (n=24), family life (n=22), security (n=25), mood (n=24), daily quality of life (n=23), self-esteem (n=25), confidence (n=26), and sensuality (n=26) Figure 3).
Discussion
Based on the literature, the BBL procedure has been in practice since the 1980’s and was introduced as a combination of liposuction and gluteal augmentation using fat transfer.
Since it has been in practice, this procedure continues to evolve and incorporate other procedures and treatments to enhance the results.
Among these procedures and treatments are liposuction of the abdomen and/or flanks, liposuction of the thighs, abdominoplasty, and helium-based plasma RF device treatment.
The ability to enhance the results of a BBL procedure by treating the surrounding body area with an alternative treatment could benefit the patients and the providers alike.
This could bring the patients closer to achieving their goals of having plastic surgery and the provider could potentially optimize their care and have more consistent and positive outcomes.
The results of this study show that patients were, in some way (slightly satisfied, satisfied, very satisfied) and for the most part (n=27), satisfied with having had the BBL procedure with heliumbased plasma RF device treatment.
Most patients (n=24) would also recommend this procedure with adjunct treatment to a friend or even consider undergoing the same procedure and treatment again themselves.
This combined with the improvements that the majority of patients (n=27) reported (skin tightness and skin appears tighter) indicates that there was a positive outcome to having the BBL procedure with helium-based plasma RF device treatment.
The results of this study also show that there was limited data indicating a need for further studies to be done to better evaluate the effects on patient satisfaction and procedural outcomes when the helium-based plasma RF device is used as an adjunct treatment to BBL procedures. Further research should focus on obtaining a more diverse patient population (age & sex), procedural data (helium-based plasma RF device settings, energy applied, aspirated amount, transferred amount, procedures performed, areas treated, etc.), safety data (AEs & ETEs during the procedure, post-operatively, and throughout follow-up), Global Aesthetic Improvement Scale assessments from both the PI and patient, and patient satisfaction data at more points throughout the patient’s follow-up care.
With further research, we could gain a deeper understanding into how the utilization of the helium-based plasma RF device as an adjunct treatment to BBL procedures is impacting patient outcomes. We can also gain an understanding as to how this device is being used in conjunction with BBL procedures, among others, and see if there is a level of standardization in practice. With more information we could provide guidance for use of the helium-based plasma RF device that standardizes the practice and positively influences patient outcomes, safety, and patient satisfaction.
Conclusion
The purpose of this study was to evaluate safety and procedural information for Brazilian Butt Lift (BBL) procedures with the helium-based plasma RF device as an adjunct treatment. A review of 49 female patients charts from one site was conducted, with 29 patients completing the Patient Satisfaction Questionnaire. Based on the available data, the helium-based plasma RF device settings for Power (%) for 31 (63%) patients was 80% and Helium Flow Rate (L/min) had 29 (94%) patients with 2.5 L/min and 2 patients (7%) with 2.0 L/ min. Most of the energy levels were not collected, but two patient charts reflected an energy level (kJ) of 6.0kJ for the abdomen, right, and left flank treatment areas. Patient satisfaction data was collected on twenty-nine (29) subjects and reported as 34% (n=10) were very satisfied, 52% (n=15) were satisfied, 7% (n=2) were slightly satisfied, 3% (n=1) were dissatisfied, and 3% (n=1) were very dissatisfied. The changes that the patients saw in the treated areas consisted of 93% (n=27) saw an improvement in skin tightness, 76% (n=22) skin feels better, and 93% (n=27) skin appears tighter. This study is limited due to the retrospective design and data availability during chart review.
Disclosures
Apyx Medical Corporation was the Sponsor of the study and provided funding for the study. Patients who previously underwent a Brazilian Butt Lift (BBL) procedure with a heliumbased plasma RF device utilized as an adjunct procedure were enrolled in the study and contacted to complete a Patient Satisfaction (PSQ). Of those enrolled and who had completed the PSQ, up to 5 were requested to return to the office for follow-up images. The study was approved by Sterling Institutional Review Board on September 10, 2021, and was conducted by Principal Investigator Alexandre S. T. De Souza, MD.
References
- Kalaaji A, Dreyer S, Vadseth L, Maric I, Jonsson V, et al. (2019) Gluteal augmentation with fat: Retrospective safety study and literature review. Aesthet Surg J 39: 292-305.
[Crossref], [Google Scholar], [Indexed]
- https://franklin.library.upenn.edu/catalog/FRANKLIN_9977967512703681
- De Souza Alexandre (2021) Brazilian Butt Lift: A clinical anatomy guide for liposuction and fat transfer. Napoleao-Quntessence Publishing Brazil.
- Fatemi A, Weiss MA, Weiss RA (2002) Short-term histologic effects of nonablative resurfacing: Results with a dynamically cooled millisecond-domain 1320nm Nd: YAG laser. Dermatol Surg 28: 172-176.
[Crossref], [Google Scholar], [Indexed]
- Hsu T, Kaminer M (2003) The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg 22: 115-123.
[Crossref], [Google Scholar], [Indexed]
- Alster TS, Doshi SN, Hpping SB (2004) Combination surgical lifting with ablative laser skin resurfacing of facial skin: a retrospective analysis. Dermatol Surg 30: 1191-1195.
[Crossref], [Google Scholar], [Indexed]
- Zelickson BD, Kist D, Bernstein E, Brown DB, Ksenzenko S, et al. (2004) Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: A pilot study. Arch Dermatol 140: 204-209.
[Crossref], [Google Scholar], [Indexed]
- Doshi SN, Alster TS (2005) Combination radiofrequency and diode laser for treatment of facial rhytides and skin laxity. Cosmet Laser Ther 7: 11-15.
[Crossref], [Google Scholar], [Indexed]
- Mayoral FA (2007) Skin tightening with a combined unipolar and bipolar radiofrequency device. J Drugs Dermatol 6: 212-215.
[Google Scholar], [Indexed]
- Ross EV, McKinlay JR, Anderson RR (1999) Why does carbon dioxide resurfacing work? A review. Arch Dermatol 135: 444-454.
[Crossref], [Google Scholar], [Indexed]
- Gardner ES, Reinisch L, Stricklin GP, Ellis DL (1996) In vitro changes in non-facial human skin following CO2 laser resurfacing: A comparison study. Lasers Surg Med 19: 379-387.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences