Cellular and Molecular Mechanism of Dermal Fibrosis Following Burn Injury, and Exploration of Therapeutic Approaches
Jie Ding and Edward E Tredget
DOI10.4172/2472-1905.100003
Wound Healing Research Lab, Department of Surgery, University of Alberta
- *Corresponding Author:
- Jie Ding
Research Associate, Wound Healing Research Lab
Department of Surgery, University of Alberta
Tel: 1-780-4920061
E-mail: jied@ualberta.ca
Received date: September 22, 2015; Accepted date: September 25, 2015; Published date: October 05, 2015
Citation: Jie Ding, Tredget EE. Cellular and Molecular Mechanism of Dermal Fibrosis Following Burn Injury, and Exploration of Therapeutic Approaches. J Aesthet Reconstr Surg. 2016, 1:3. doi: 10.4172/2472-1905.10003
Editorial
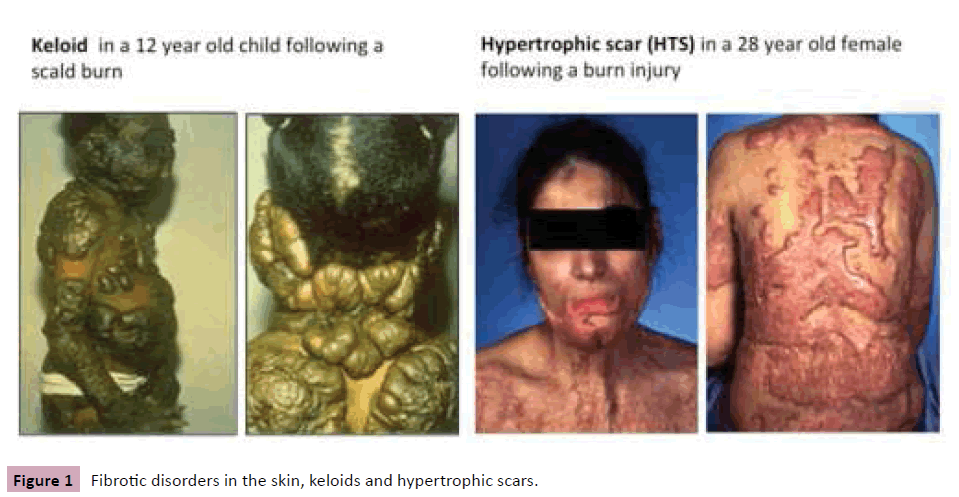
Fibrotic disorders such as liver cirrhosis, pulmonary fibrosis, systemic sclerosis, valvular disease, atherosclerosis, etc. are defined by overgrowth, hardening and scarring of various tissues. The majority of these diseases are progressive, irreversible and fatal, and they are responsible for the deaths of millions of people. There are two fibrotic disorders in the skin, keloids and hypertrophic scars (HTS) (Figure 1). Clinically, the most important difference between keloids and HTS, is that keloids develop beyond the borders of the original wound and tend to recur after excision, whereas HTS never expand beyond the boundaries of the initial wound and may partially spontaneously regress. HTS occurs after deep dermal burn injury with prolonged inflammation. Our research group focuses on the cellular and molecular mechanism of dermal fibrosis following burn injury, and the exploration of therapeutic approaches.
Skin is the largest organ of the body with 3 layers: epidermis, dermis, and hypodermis. It provides a vital barrier function, plays important roles in the wound healing process, and contributes to thermoregulation of our bodies. Depending on the depth of tissue damage, burns are classified clinically into 4 degrees: 1st degree, superficial or deep 2nd degree, 3rd degree full thickness, and 4th degree burns. Wound healing is an intricate process, in which the skin repairs itself after injury. The normal process of wound healing has four phases. The hemostatic phase is from initial injury to 3 hours, in which blood vessels constrict, clot forms and cytokines are released from platelets. The inflammatory phase is from 0 to 3 days. During this time, leukocytes and macrophages infiltrate to the wound site, clean the wound and destroy the bacterial and cell debris. The proliferation phase is from 3 days to 3 weeks where blood vessels and granulation tissue start to form to fill the wound cavity. Finally, the remodelling phase is from 3 weeks to 1 year. The disorganized collagen fibres are rearranged, cross-linked, and aligned under the direction of remodelling [1].
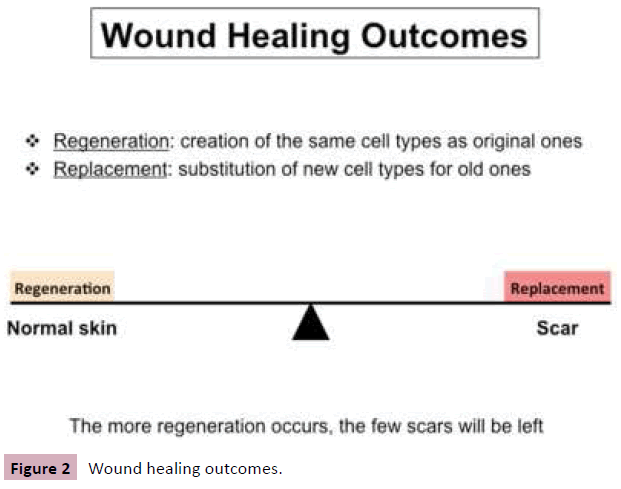
Wound healing is often a mixture of regeneration and replacement. Regeneration occurs when wounds heal with the same cell types as originally present; replacement occurs when the wound heals with new cell types. The wound healing outcomes are easily understood using the “Fence Theory” analogy [2]. Burn injury makes a wound in the skin. Regenerationbased wound healing is a normal process of wound repair, and restores normal skin; however, replacement-based wound healing creates abnormal wound repair, causing scars including mature scars or abnormal scars. Mature scars are flat, pale, soft, insensitive, and leave a trace on the body. Abnormal scars include keloids and HTS. The more regeneration that occurs, the fewer scars which will be left (Figure 2). In morphology, HTS are red, raised, uncomfortable scars confined to the boundaries of the original wounds, which can result in functional limitations due to the development of contracture and disfigurement and also lead to cosmetic problems for burn survivors. In histology, compared to normal skin, HTS are thicker in the epidermis and dermis, lack rete ridges in the epidermis, have increased vascularity, increased cellularity including macrophages, mast cells, T cells, fibrocytes, fibroblasts and myofibroblasts, atypical extracellular matrix (ECM) remodeling, high expression of transforming growth factor beta (TGFβ) and low expression of decorin in the dermis. Some forms of treatment used in patients include surgical, nonsurgical, and immunologic and biomolecular treatments. However, no method so far can prevent and replace the abnormal scars completely. Therefore improved understanding may offer exciting therapy for HTS and other fibrotic disorders.
Our research group has made some achievements in the following aspects related to hypertrophic scarring in the past two decades.
1. Establishment of dermal fibrotic mouse models
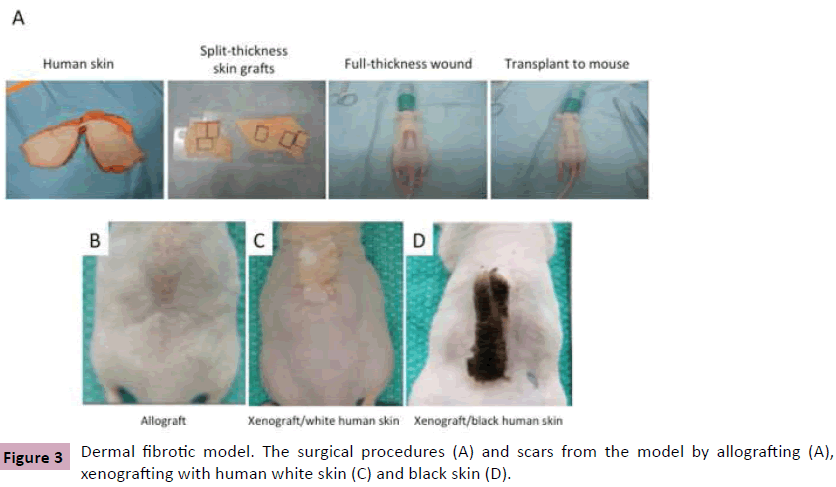
A key challenge to improve our understanding of HTS is the discovery of a relevant animal model. Several animal models have been described in the literatures each with their own limitations. Based on our experience, the nude mouse model represents a promising animal model. The nude mouse is homozygous null for the Foxn1 gene, resulting in a deficiency of T cells, which allows transplanting human skin onto mouse. In 2007, Yang et al reported a nude mouse model by grafting full-thickness human skin [3]. In 2011, we modified it and established our own nude mouse model by grafting split-thickness human skin. The surgical procedures include: 1) Collection of human skin from patients undergoing abdominoplasty. 2) Preparation of split-thickness skin grafts from it. 3) Preparation of a full-thickness excisional wound on the back of the nude mouse. 4) Transplantation of human skin graft onto the mouse (Figure 3A). In morphology, compared to the control transplanted with mouse skin (Figure 3B), the mouse grafted with split-thickness human skin developed thick scar at 1 month after grafting, consistent with human HTS (Figure 3C). In histology, we found reduced hair follicles and rete ridges, increased cells and blood vessels, thin collagen fiber accumulation parallel to the skin surface, elevated TGFβ and reduced decorin, and recruitment of macrophages, mast cells, fibrocytes and myofibroblasts. To confirm that human skin survives the wound healing process, black human skin was grafted onto mouse and black scar developed (Figure 3D) [4-6].
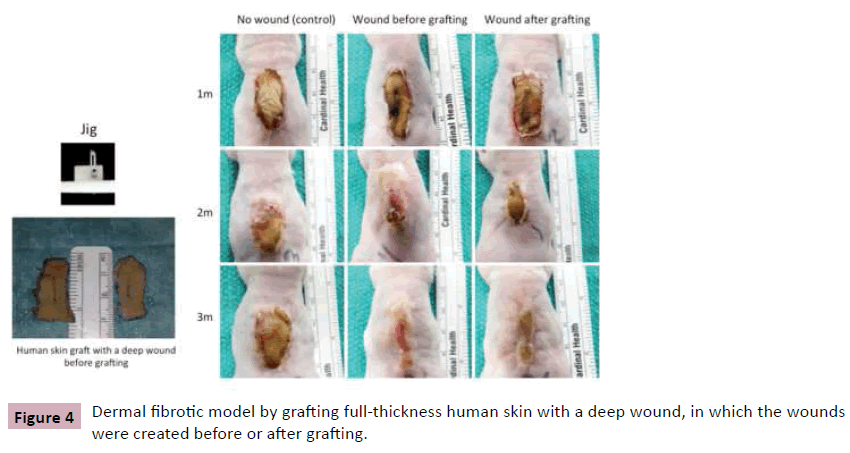
Besides nude mice, scars also developed in RAG1, RAG2 and TCR knockout mice, in which mature T, B, NK cells and T cell receptors are deficient, suggesting that non-specific immune cells such as macrophages and mast cells may play a significant role in this dermal fibrotic model [7]. The roles of non-specific immune cells in wound healing and scarring have being studied now. Based on this model, we further modified the mouse model by grafting full-thickness human skin with a deep wound, in which the deep wound was created using a jig before or after grafting, which may create a more promising dermal fibrotic mouse model (Figure 4) [6].
2. Involvement of chemokine pathways in hypertrophic scarring
The recruitment of blood-borne cells (macrophages, mast cells, fibrocytes) in HTS tissues suggests a possible role of chemokines, which are named for their ability to attract cells from one place to another. Stromal cell-derived factor-1 (SDF-1) has been found a potent chemoattractant for lymphocytes and monocytes. Stromal cells are connective tissue cells of any organ. Fibroblasts and pericytes are the most common types of stromal cells, which produce SDF-1. SDF-1 is induced by proinflammatory factors, and functions by binding to CXCR4. Recently it has been suggested this pathway plays a role in fibrotic diseases of lung, liver and kidney. SDF-1 is elevated in human HTS tissue compared to site-matched normal skin from recovering burn patients. Of the peripheral blood mononuclear cells (PBMCs) in burn patients, the population of CD14+CXCR4+ cells is higher compared to controls, particularly the CD14+hiCXCR4+ cells. Serum SDF-1 in burn patients positively correlates with total body surface area, but negatively correlates with age. Interferons (IFNs) are antifibrotic factors that have been used in various fibrotic conditions. In our phase II clinical trial, burn patients, who had developed severe HTS, were treated with IFNα2b, which significantly improved scarring in 7 of 9 patients [8]. IFN2b down-regulated SDF-1 in HTS tissues compared to the tissues before the treatment. Reduced population of CD14+hiCXCR4+ was found in PBMCs of IFNα2b treated patients time-dependently. However, in control group, the population keeps increasing [9]. We speculated that SDF-1/CXCR4 signalling is involved in the development of HTS by promoting migration of CXCR4-expresing monocytes from the bloodstream to wound sites, where they may differentiate into fibrocytes, fibroblasts and myofibroblasts. If this is correct, blocking the SDF-1/CXCR4 pathway will prevent the homing of CXCR4-expressing monocytes from the circulation into wounds and minimize HTS formation. To confirm it, CTCE-9908, a small peptide analog of SDF-1, was used in the following study as a CXCR4 antagonist. By in vitro transwell migration assay, both rhSDF-1 and lipopolysaccharide (LPS)-induced fibroblast supernatant induced PBMC migration. CXCR4 neutralization antibody completely blocked the PBMC migration induced by rhSDF-1, which was used as a positive control. CTCE-9908 blocked much of the PBMC migration induced by rhSDF-1 or fibroblast conditioned medium. Using the dermal fibrotic nude mouse model, human skin grafts in the control mice started to contract and eventually hypertrophic, firm, and raised scar developed 8 weeks after grafting. Grafts on the CTCE-9908 injected mice remained flat with limited contraction. Histologically, CTCE-9908 reduced the scar thickness, cellularity and vascularity, improved collagen remodeling, decreased fibrotic gene expression, reduced the recruitment of macrophages and myofibroblasts, down-regulated pro-fibrotic factor TGFβ1, and up-regulated anti-fibrotic factor decorin [10]. The study offers a potential therapy for patients with dermal fibrotic conditions after burns and other forms of trauma to the skin.
3. More fibrotic features of deep dermal fibroblasts
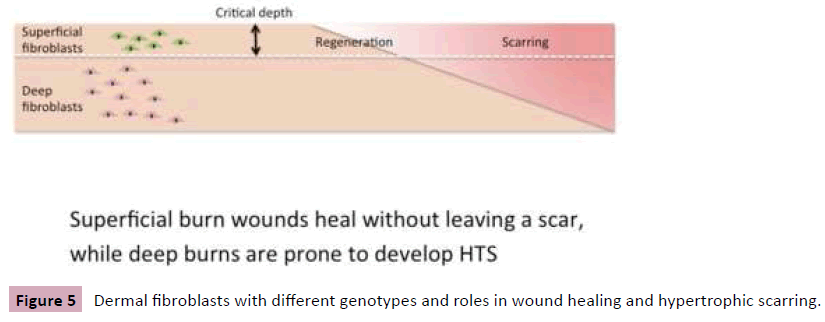
Clinically, superficial burn wounds heal without leaving a scar, while deep burns are prone to develop HTS. It is our hypothesis that deep dermal fibroblasts are more fibrotic and contribute to hypertrophic scarring (Figure 5) [11]. To confirm this, superficial and deep burn wounds (SW and DW) were collected from burn patients, where Laser Doppler Imaging was performed for determination of depth of the wounds [12]. DW have more fibrocytes, express more fibrotic factors at gene and protein level compared to SW. In a clinical study, we made scratched-incision wounds in the skin of volunteers using a jig, in which the wound is progressively deeper from one end to another. The wounds healed with mature scar on the superficial side and HTS on the deeper side. In vitro, superficial and deep fibroblasts (SF and DF) were cultured from superficial and deep dermis from patients underwent abdominoplasty. DF are bigger in size, grow slowly in culture, express more fibrotic factor genes, and less MMP1 (collagenase). DF produce more collagen and TGFβ1, induce a stronger gel contraction compared to SF. DF migrate slow, and express less decorin but more biglycan. Decorin up-regulates more apoptosis genes in SF, but down-regulates their expression in DF [13-15]. Superficial and deep dermal fibroblasts have different behaviors in wound healing and hypertrophic scarring, which is supported by the study recently published in Science [16].
4. Decorin as a target for the therapy of dermal fibrosis
Decorin is a small leucine-rich repeat proteoglycan involved in collagen fibrillogenesis by binding to type I collagen fibrils. It is thought to be an anti-fibrotic factor based on multiple in vivo and in vitro experiments. Over the past few years, the roles of epigenetic modifications caused by environmental factors have been intensively studied in relation to disease pathogenesis. miRNAs are a group of small non-coding RNA molecules that can regulate gene functions. Decorin was found lower in HTS, DW, burned tissue and DF compared to NS, SW, unburned tissue and SF. By mass screening, we found DF express more of miRNA 24, 181b and 421, which are thought to regulate decorin. Decorin was mainly down-regulated by miRNA-181b, and promoting decorin gene expression by transfecting a plasmid with decorin gene or blocking miRNA-181b maybe a potential therapy for HTS [17]. In vitro, decorin upregulated toll like receptor 4 and CXCR4 expression in PBMCs [18]. We are working on the role of decorin in dermal fibroblasts and 3D skin substitute model, and exploring the therapeutic potential for HTS.
5. Preparation of engineered skin substitutes for exploration of the biological events involved in wound healing and dermal fibrosis
Although modern medicine provides many effective treatments, damaged organs frequently still cannot be returned to full health. Tissue engineering therefore is being developed. Early in 1993, a definition was stated by Langer: Tissue engineering is an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ [19]. Skin autografts are often used for patients due to skin loss resulting from burns or other skin injury. However, in the case of extensive skin loss, autografts are not available and therefore engineered skin substitutes (ESS) are the preferred treatment for wound closure. In our lab, we have successfully produced ESS for the exploration of the biological events involved in wound healing and dermal fibrosis by co-culture of human fibroblasts, keratinocytes and artificial collagen-glycosaminoglycan (C-GAG) matrix. ESS cultured with DF have negative mechanical features of higher stiffness and lower ultimate tensile strength, contracted more, contained higher amounts of collagen, and induced more myofibroblast formation, suggesting differential C-GAG matrix remodeling by SF and DF cells. SF increase epidermal barrier function and formed better basement membranes in ESS. Keratinocytes improved fibrotic remodeling, biomechanicalcharacteristics and pore microstructure of ESS with DF [20-23]. All these results suggest that remodeling of C-GAG matrix is regulated by layered dermal fibroblasts and keratinocytes, which is a potential therapeutic target for HTS.
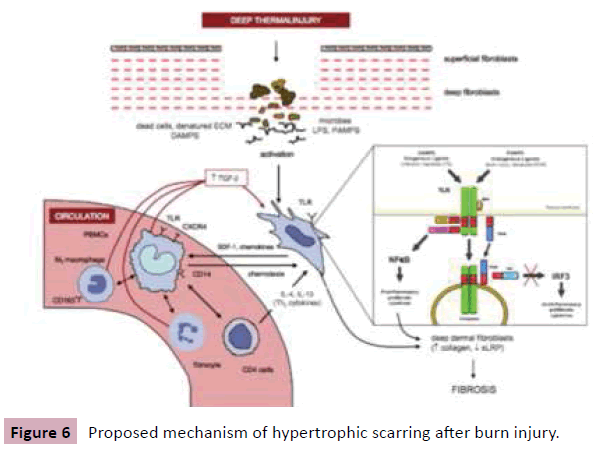
So far, we understand that deep burn injury activates deep dermal fibroblasts through toll-like receptors by inflammatory signals derived from infectious pathogens like LPS and endogenous molecules including denatured ECM. Then activated fibroblasts, in turn, release chemokines such as SDF-1, which lead to the recruitment of blood-borne cells into wounds. These cells contribute to hypertrophic scarring by differentiating into fibroblasts and myofibroblasts, or regulating fibroblasts towards fibrosis by multiple growth factors such as TGFβ (Figure 6) [15]. Of course, the current understanding of hypertrophic scarring will continue to be revised through further research in future.
References
- Beanes SR, Dang C, Soo C, Ting K (2003) Skin repair and scar formation: the central role of TGF-beta. Expert Rev Mol Med 5: 1-22.
- Tredget EE (1999) Pathophysiology and treatment of fibroproliferative disorders following thermal injury. Ann N Y AcadSci 888: 165-182.
- Yang DY, Li SR, Wu JL, Chen YQ, Li G, et al. (2007) Establishment of a hypertrophic scar model by transplanting full-thickness human skin grafts onto the backs of nude mice. PlastReconstrSurg 119: 104-109.
- Wang J, Ding J, Jiao H, Honardoust D, Momtazi M, et al. (2011) Human hypertrophic scar-like nude mouse model: characterization of the molecular and cellular biology of the scar process. Wound Repair Regen 19: 274-285.
- Momtazi M, Kwan P, Ding J, Anderson CC, Honardoust D, et al. (2013) A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen 21: 77-87.
- Alrobaiea SM, Ding J, Ma Z, Tredget EE, A Novel Nude Mouse Model of Hypertrophic Scarring Using Scratched Full-Thickness Human Skin Grafts (Accepted by Advances in Wound Care).
- Momtazi M, Ding J, Kwan P, Anderson CC, Honardoust D, Morphologic and Histological Comparison of Hypertrophic Scar in Nude Mice, Tcell Receptor and Recombination Activating Gene (RAG) Knockout Mice (Accepted by Plastic and Reconstructive Surgery).
- Tredget EE, Shankowsky HA, Pannu R, Nedelec B, Iwashina T, et al. (1998) Transforming growth factor-beta in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. PlastReconstrSurg 102: 1317-1328.
- Ding J, Hori K, Zhang R, Marcoux Y, Honardoust D, et al. (2011) Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS). Wound Repair Regen 19: 568-578.
- Ding J, Ma Z, Liu H, Kwan P, Iwashina T, et al. (2014) The therapeutic potential of a C-X-C chemokine receptor type 4 (CXCR-4) antagonist on hypertrophic scarring in vivo. Wound Repair Regen 22: 622-630.
- Kwan P, Hori K, Ding J, Tredget EE (2009) Scar and contracture: biological principles. Hand Clin 25: 511-528.
- Stewart TL, Ball B, Schembri PJ, Hori K, Ding J, et al. (2012) The use of laser Doppler imaging as a predictor of burn depth and hypertrophic scar postburn injury. J Burn Care Res 33: 764-771.
- Wang J, Dodd C, Shankowsky HA, Scott PG, Tredget EE; Wound Healing Research Group (2008) Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest 88: 1278-1290.
- Honardoust D, Ding J, Varkey M, Shankowsky HA, Tredget EE (2012) Deep dermal fibroblasts refractory to migration and decorin-induced apoptosis contribute to hypertrophic scarring. J Burn Care Res 33: 668-677.
- Ding J, Ma Z, Shankowsky HA, Medina A, Tredget EE (2013) Deep dermal fibroblast profibrotic characteristics are enhanced by bone marrow-derived mesenchymal stem cells. Wound Repair Regen 21: 448-455.
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, et al. (2015) Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151.
- Kwan P, Ding J, Tredget EE (2015) MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLoS One 10: e0123054.
- Kwan PO, Ding J, Tredget EE (2015) Serum Decorin, IL-1β, and TGF-β Predict Hypertrophic Scarring Postburn. J Burn Care Res.
- Langer R1, Vacanti JP (1993) Tissue engineering. Science 260: 920-926.
- Varkey M, Ding J, Tredget EE (2011) Differential collagen-glycosaminoglycan matrix remodeling by superficial and deep dermal fibroblasts: potential therapeutic targets for hypertrophic scar. Biomaterials 32: 7581-7591.
- Varkey M, Ding J, Tredget EE (2014) Superficial dermal fibroblasts enhance basement membrane and epidermal barrier formation in tissue-engineered skin: implications for treatment of skin basement membrane disorders. Tissue Eng Part A. 20: 540-552.
- Varkey M, Ding J, Tredget EE (2014) Fibrotic remodeling of tissue-engineered skin with deep dermal fibroblasts is reduced by keratinocytes. Tissue Eng Part A 20: 716-727.
- Varkey M, Ding J, Tredget EE (2014) Wound Healing Research Group:The effect of keratinocytes on the biomechanical characteristics and pore microstructure of tissue engineered skin using deep dermal fibroblasts. Biomaterials 35: 9591-9598.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences