Enterococcus Breast Implant Infections: A Case Report and Review of Literature
Anna Tanaka1*, Stephanie Wythe2, Ryan Miller3, Sarah N. Bishop2
1Department of Plastic and Reconstructive Surgery, Case Western Reserve University School of Medicine, Cleveland, United States
2Department of Plastic and Reconstructive Surgery, Cleveland Clinic Foundation, Cleveland, United States
3Department of Infectious Disease, Cleveland Clinic Foundation, Cleveland, United States
- *Corresponding Author:
- Anna Tanaka
Department of Plastic and Reconstructive Surgery,
Case Western Reserve University School of Medicine, Cleveland,
United States,
E-mail: axt622@case.edu
Received date: January 19, 2023, Manuscript No. IPARS-22-15658; Editor assigned date: January 20, 2023, PreQC No. IPARS-22-15658 (PQ); Reviewed date: February 7, 2023, QC No. IPARS-22-15658; Revised date: February 10, 2023, Manuscript No. IPARS-22-15658 (R); Published date: February 17, 2023, DOI: 10.36648/2472-1905.9.1.155
Citation: Tanaka A, Wythe S, Miller R, Bishop SN (2023) Enterococcus Breast Implant Infections: A Case Report and Review of Literature. J Aesthet Reconstr Surg Vol.9 No.1: 155.
Abstract
Introduction: Periprosthetic breast implant infections are pervasive and serious complications of implant- based breast reconstruction that continues to present a challenge for plastic surgeons. Breast implant infections due to common causative bacteria such as Staphylococcus spp. and Pseudomonas spp. have been well-described in the literature; however, there are very few reported cases of Enterococcus spp. implant infections, leaving a current gap in knowledge on effective management strategies. There is a concern that indolent Enterococcus infections can lack clinical symptoms but ultimately disseminate and progress to endocarditis.
Case Report: We report an unusual case of an insidious bilateral breast implant infection with Enterococcus faecalis in a 51-year-old female. The patient underwent bilateral implant-based breast reconstruction with pre-pectoral tissue expanders and Acellular Dermal Matrix (ADM) following nipple sparing mastectomies. This patient lacked all clinical features of infection during her postoperative course, including fever, erythema, swelling, and pain. During exchange of her tissue expanders for permanent implants, a physiologic amount of serous but viscous, periprosthetic fluid was encountered in each breast pocket, subsequently identified as an indolent E. faecalis infection. This patient ultimately underwent removal of her bilateral implants and six weeks of antimicrobial therapy with delayed reconstruction six months later.
Conclusions: This case emphasizes that not all implant infections present with clear, observable signs of infection. High clinical suspicion is needed given the severity of consequences of a missed diagnosis including eventual reconstructive failure, but also hematogenous dissemination, infectious endocarditis, and other life-threatening disease. We recommend an aggressive treatment approach with implant removal and an extensive course of targeted antimicrobial therapy to minimize the risk of these untoward complications.
Keywords
Breast implant; Acellular Dermal Matrix; Antimicrobial; Periprosthetic
Introduction
Breast implant infections are a common post-surgical complication following breast augmentation and reconstruction procedures. According to the plastic surgery statistics report, in 2020 there were a total of 137,808 breast reconstruction procedures done, and of these, 22,676 implant removals were performed [1]. The incidence of breast implant infections is reported to be as high as 20% [2,3], indicating that these pervasive infections continue to present a challenge for plastic surgeons. Breast implant infections can lead to a multitude of adverse physical and psychological outcomes for the patient including a more prolonged hospital stay, readmission, implant loss, delayed overall treatment of cancers, and compromise of aesthetic outcomes [2,4]. In particular, breast implant infections related to reconstruction after mastectomy occur as high as 10 times as often compared to implants for augmentation, thought to be due to skin atrophy and tissue scarring from radiation therapy [5,6]. Other well-known risk factors for infection include having a high BMI, use of drains, smoking, comorbid diabetes mellitus, use of acellular dermal matrix, chemotherapy, radiation therapy, and immediate reconstruction [2,5]. Given the high prevalence and harmful impact of these infections, proper diagnosis and effective management of breast implant infections are crucial in minimizing burden on both the patient and the healthcare system.
The majority of the causative organisms in breast implant infections include Gram-positive organisms such as Staphylococcus aureus (including MRSA) and Staphylococcus epidermidis, as well as Gram negative species such as Pseudomonas aeruginosa [7,8]. Although less common, species such as Serratia, Enterococcus, Enterobacter, Streptococcus, and Morganella have also been associated with breast implant infection [8]. The origin of implant infection is difficult to determine but can include a contaminated implant, contamination during surgery, the patient’s skin or breast ducts, as well as seeding of the implant from a remote infection [9].
In terms of clinical presentation, breast implant infections present in a bimodal manner. Early-onset infections emerge on average 10 days post-surgery, and typically within the first 6 weeks after surgery. Meanwhile, sub-acute indolent bacterial infections usually present several months after implantation [5,7]. Late infection, months to years after implantation, can also occur secondary to bacteremia or an invasive procedure at a location other than breasts, where the implantation site “acts as a trap for bacteria” [9]. It is also possible that late implant infections are due to indolent species from the original procedure presenting at a very delayed time. The typical clinical presentation of early-onset infections consists of breast pain, swelling, erythema, fever, and purulent fluid or drainage at the incision site [5,7,10]. In contrast, indolent infections may present with more focal signs such as a non-healing surgical site, incisional drainage, dehiscence, or extrusion of the implant [5].
The presence of clear, observable symptoms seen with early-onset infections makes these infections relatively easy to identify with routine monitoring of the breasts after surgery. However, not all breast implant infections present with these classical signs of infection some infections can be extremely subtle, with no clear symptoms. This makes the diagnosis of indolent implant infections to be more challenging. In the following case report and summary of the present literature, we describe the presentation, clinical course, and management of a case of a subclinical Enterococcus implant infection. We aim to shed light on and increase clinical awareness of indolent implant infections that have the potential to cause drastic complications of dissemination and endocarditis if left undiagnosed.
Case Presentation
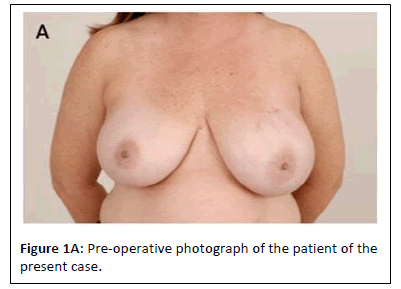
Our patient is a 51-year-old female who was diagnosed with multifocal high grade left breast Ductal Carcinoma in Situ (DCIS). She underwent bilateral nipple sparing mastectomy and bilateral immediate breast reconstruction with prepectoral tissue expanders and acellular dermal matrix (AlloDerm, Allergan, New Jersey). Her immediate postoperative course was complicated by right mastectomy flap skin malperfusion which was successfully treated with nitroglycerin paste(Figure 1A).
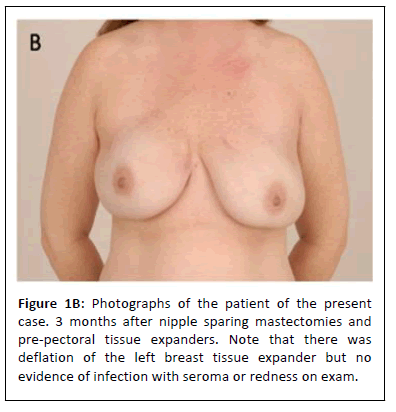
She was seen in the clinic on post-operative days six, twelve, and twenty. She was noted to be healing well with no signs or symptoms of infection, wound breakdown, or mastectomy flap necrosis. She underwent routine postoperative wound care, and her drains were removed when their output was appropriate. Three months postoperatively, it was noted that her bilateral breast tissue expanders had deflated, but she was happy with her size and wished to proceed with implant exchange (Figure 1B).
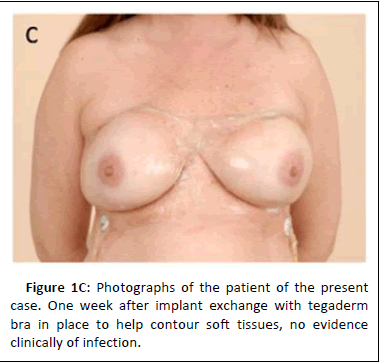
The patient subsequently underwent bilateral breast tissue expander exchange for silicone breast implants. At the time of implant exchange, 5 to 10 ccs of clear, non-purulent, physiologic-appearing fluid was encountered in each periprosthetic pocket. The patient did not have any symptoms of infection or any skin findings consistent with infection (Figure 1C).
This fluid was sent for culture and implant exchange proceeded as planned. Four days later, fluid cultures grew Enterococcus faecalis and the patient was referred to an infectious disease specialist.
The patient was seen by Infectious Disease and started on culture-guided antimicrobial therapy with oral amoxicillin. Infectious disease noted that Enterococcus spp. tends to form biofilms and cause insidious, smoldering infections that may not become clinically robust until the organism has disseminated from the source. Ultimately, dissemination of the infection could lead to endocarditis. Due to the indolent course, the typical clinical signs of infection, swelling, and increased fluid collection would not necessarily be seen. Multiple shared decision-making conversations were had between the patient, infectious disease, and the attending plastic surgeon. It was ultimately decided to treat this infection aggressively with implant removal and long-term antimicrobial therapy. We had considered using serial ultrasounds to look for fluid accumulation; however, this patient did not have a seroma or increased fluid noted around the implant so this may not be a reliable marker. Five days after the positive culture result, the patient underwent bilateral breast implant removal with irrigating wound vac placement. Additional cultures were taken at this time, which eventually grew Staphylococcus epidermidis and no growth of Enterococcus. The patient was treated in the hospital for three days with 0.25% Dakin's solution irrigations via the wound vac and intravenous ampicillin. She was then taken back to the operating room for vac removal and flat closure over drains. She was discharged from the hospital and treated with a four-week course of amoxicillin and doxycycline.
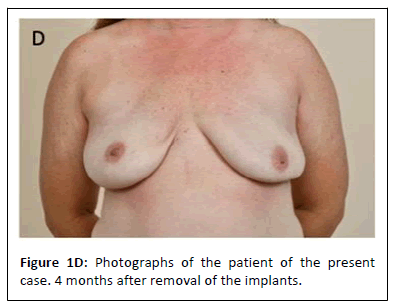
She was followed closely as an outpatient and she completed her course of antibiotics. Her incisions healed well and she did not develop any signs of infection (Figure 1D).
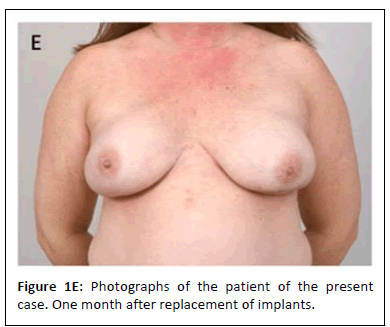
Approximately six months later, she underwent repeat bilateral breast reconstruction with silicone breast implants and skin envelope revision for shape and symmetry. This time, the breast pockets appeared normal with no intra-capsular fluid. Tissue was sampled for culture, which revealed no growth. Since this operation, her recovery has been uneventful and she is happy with her results (Figure 1E).
Discussion
Enterococcus implant infections are an uncommon culprit of breast implant infections. As a result, there are few to no detailed descriptions of Enterococcus implant infections reported in the literature. Our case was unique from typical implant infections in that the patient did not present with any overt clinical signs of infection. The patient never had any breast erythema, swelling, seroma, wound healing issues, fever, or pain. This made this case distinct from the large majority of reports where the patient presents with at least one or more of the key signs of infection. Furthermore, intraoperatively, the AlloDerm was noted to be incorporated, along with approximately 5-10 milliliters of fluid which is physiologic and seen with most implants [11]. The only indication of infection was that the fluid, albeit clear and non-purulent in appearance, was noted to subjectively have a viscous quality to it. Without a cautionary bacterial culture sample taken, the infection likely would have been missed until a much later time.
Of the scant reports on Enterococcus breast implant infection, there has been a reported case of Enterococcus avium infection that occurred 16 years after breast implantation thought to be secondary to a bloodstream infection, with the patient presenting with acute onset of swelling, pain, and erythema [12]. Other indolent breast implant infections reported have been caused by Mycobacterium, presenting 54 days post-surgery with a subtle presentation of mild pain and swelling, but without fever or erythema[7]. Unlike our case, the reported patient presented with a few signs of infection which led to the identification of the infection. Within the existing literature, we were unable to find any other similar reported cases of Enterococcus breast implant infection that presented as covertly as it did in our case.
Early identification of breast implant infection is clinically significant because of the nature of the consequences if left undiagnosed. Severe complications can include life-threatening consequences such as toxic shock syndrome, chronic rib osteomyelitis, and endocarditis [13]. Enterococcus as a species is a biofilm-forming bacteria that is difficult to eradicate, and in this case, although uncommon, concern for dissemination of the infection and resultant endocarditis was our main concern. Once disseminated, the risk of endocarditis is not negligible. Enterococci can adhere to and infect both normal and previously damaged valves [14] and are the third most common cause of infective endocarditis [15]. In the study by Anderson et al., 8-32% of patients with Enterococcal bacteremia subsequently developed Enterococcal endocarditis [15].
Further, because Enterococci are natural inhabitants of the genitourinary and gastrointestinal tracts, trauma, procedures, and surgical implants to this region are known to be potential sources for invasion that lead to Enterococcal endocarditis [14]. In the literature, multiple cases of Enterococcal endocarditis have been reported to be caused by a variety of GI and GU procedures such as prostatic urethral lifts and colonic polyp resection [16-18]. Enterococcal endocarditis is associated with significant morbidity and mortality, as therapy can be difficult due to antibiotic resistance [15]. The impact of a missed Enterococcus implant infection can be as severe as congestive heart failure and the need for a valve replacement procedure.
In the orthopedic literature, Enterococcal prosthetic joint infections have been more robustly described [19,20] and have been shown to have high rates of treatment failure and complications [21]. Although it is often difficult to pinpoint the primary source of infection, there has been a reported case of Enterococcal endocarditis that was thought to be secondary to infection of bilateral knee prostheses [22]. Beyond Enterococci, in one study of 166 patients with Staphylococcus aureus bacteremia due to prosthetic joint infection, 3 patients developed endocarditis [23]. Although infrequent, due to the high volume of breast implant procedures performed, the risk of implant infection and subsequent dissemination of infection cannot be overlooked.
Finally, due to the indolent nature of bacteria, patients with an Enterococcus infection typically will not have any symptoms until the infection brews and advances to the point of dissemination. Even for patients that progress to endocarditis, symptoms tend to be unspecific, with only moderately elevated white blood cell count and often without a systemic inflammatory response syndrome despite having a severe infection with extensive intra-cardiac infiltration [24]. Compared to other bacteria, symptoms from Enterococcal endocarditis tend to be more sub-acute [14].
Within the GI epithelium, E. faecalis can “hide in plain sight” and remain undetected in asymptomatic patients to provide an infectious reservoir for endocarditis to present at a much later time [25]. In the report by Tracy et al., the patient initially presented with a non-specific presentation and lack of typical stigmata, highlighting that diagnosis of Enterococcal endocarditis is difficult and often delayed [22]. Overall, awareness of, and early identification of these infections is critical for the patient. This is in stark contrast to the more common Staphylococcus infections that are significantly less likely to progress to such severity due to the early manifestation of clinical symptoms.
Conclusion
Enterococcus breast implant infections are fortunately rare. If detected, however, these infections require unique management. We recommend an aggressive course to prevent dissemination of the infection. Our case report highlights the need for awareness and caution of indolent implant infections that may not present with obvious clinical features of infection. For an elective surgery such as a breast implant procedure, the patient’s safety should the highest priority.
References
- ASPS National Clearinghouse of Plastic Surgery Procedural Statistics (2020) Plastic Surgery Statistics Report. Department of Plastic Surgery.
- Boustany AN, Elmaraghi S, Agochukwu N, Cloyd B, Dugan AJ et al. (2018) A breast prosthesis infection update: Two-year incidence, risk factors and management at single institution. Indian J Plast Surg 51: 7-14.
[Crossref], [Google Scholar], [Indexed]
- Feldman EM, Kontoyiannis DP, Sharabi SE, Lee E, Kaufman Y et al. (2010) Breast implant infections: Is cefazolin enough? Plast Reconstr Surg 126: 779-785.
[Crossref], [Google Scholar], [Indexed]
- Liu EH, Tong M, Kim GY, Farrokhyar F, Dal CA (2022) Antibiotic prophylaxis in alloplastic breast reconstruction: Regimens and outcomes. Plast Surg (Oakv) 30: 25-31.
[Crossref], [Google Scholar], [Indexed]
- Lalani T (2018) Breast implant infections: An update. Infect Dis Clin North Am 32: 877-884.
[Crossref], [Google Scholar], [Indexed]
- Brand KG (1993) Infection of mammary prostheses: A survey and the question of prevention. Ann Plast Surg 30: 289-295.
[Crossref], [Google Scholar], [Indexed]
- Zhang R, Singh D, Parsa FD (2022) Review of early signs of breast implant infection. Aesthetic Plast Surg 46: 2152-2158
[Crossref], [Google Scholar], [Indexed]
- Cohen JB, Carroll C, Tenenbaum MM, Myckatyn TM (2015) Breast implant-associated infections: The role of the national surgical quality improvement program and the local microbiome. Plast Reconstr Surg 136: 921-929.
[Crossref], [Google Scholar], [Indexed]
- Pittet B, Denys M, Didier P (2005) Infection in breast implants. Lancet Infect Dis 5: 94-106.
[Crossref], [Google Scholar], [Indexed]
- Mesa F, Sebastian C, Oscar T (2021) Study of infections in breast augmentation surgery with implants in 9,691 patients over 5 years. Plast Reconstr Surg Glob Open 9: e3752.
[Crossref], [Google Scholar], [Indexed]
- Clemens MW, Eric DJ, Steven MH (2019) 2019 NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J 39: S3-13.
[Crossref], [Google Scholar], [Indexed]
- Ablaza VJ, LaTrenta GS (1998) Late infection of a breast prosthesis with enterococcus avium. Plast Reconstr Surg 102: 227-230.
[Crossref], [Google Scholar], [Indexed]
- Seng P, Bayle S, Alliez A, Romain F, Casanova D et al. (2015) The microbial epidemiology of breast implant infections in a regional referral centre for plastic and reconstructive surgery in the south of France. Int J Infect Dis 35: 62-66.
[Crossref], [Google Scholar], [Indexed]
- Megran DW (1992) Enterococcal endocarditis. Clin Infect Dis 15: 63-71.
[Crossref], [Google Scholar], [Indexed]
- Anderson DJ, Murdoch DR, Sexton DJ, Reller LB, Stout JE et al. (2004) Risk factors for infective endocarditis in patients with enterococcal bacteremia: A case-control study. Infection 32: 72-77.
[Crossref], [Google Scholar], [Indexed]
- Chidurala S, Manikumar B (2022) Unusual presentation of infective endocarditis following a prostatic urethral lift. Cureus 14: e26919.
[Crossref], [Google Scholar], [Indexed]
- Wehbeh A, Myron CG, Douglas KR (2019) Enterococcus faecalis endocarditis after endoscopic mucosal resection of a large sessile colonic polyp. ACG Case Rep J 6: e00020.
[Crossref], [Google Scholar], [Indexed]
- Irani J, Roblot F, Becq GB, Dore B (2002) Acute bacterial endocarditis secondary to transrectal ultrasound-guided prostatic biopsy. Scand J Urol Nephrol 36: 156-157.
[Crossref], [Google Scholar], [Indexed]
- Renz N, Rihard T, Doruk A, Carsten P, Andrej T (2019) Enterococcal periprosthetic joint infection: clinical and microbiological findings from an 8-year retrospective cohort study. BMC Infect Dis 19: 1083.
[Crossref], [Google Scholar], [Indexed]
- Tornero E, Senneville E, Euba G, Petersdorf S, Rodriguez PD et al. (2014) Characteristics of prosthetic joint infections due to Enterococcus sp. and predictors of failure: A multiâ?ÂÂÂÂÂÃÂnational study. Clin Microbiol Infect 20: 1219-1224.
[Crossref], [Google Scholar], [Indexed]
- Kheir MM, Timothy LT, Carlos H, Jaiben G, Craig JD et al. (2017) Periprosthetic joint infections caused by enterococci have poor outcomes. J Arthroplasty 32: 933-947.
[Crossref], [Google Scholar], [Indexed]
- Tracy SI, Sherry AB, John TR, Anjali B (2016) Rare case of simultaneous enterococcal endocarditis and prosthetic joint infection. BMJ Case Rep 2016.
[Crossref], [Google Scholar], [Indexed]
- Tande AJ, Bharath RP, Douglas RO, Elie FB, Larry MB et al. (2016) Clinical presentation, risk factors, and outcomes of hematogenous prosthetic joint infection in patients with staphylococcus aureus bacteremia. Am J Med 129: 221.e11-221.e20.
[Crossref], [Google Scholar], [Indexed]
- Dahl A, Kasper I, Niels T, Nis H, Magnus A et al. (2019) Prevalence of infective endocarditis in enterococcus faecalis bacteremia. J Am Coll Cardiol 74: 193-201.
[Crossref], [Google Scholar], [Indexed]
- Barnes AMT, Kristi LF, Gary MD (2021) Enterococcal Endocarditis: Hiding in Plain Sight. Front Cell Infect Microbiol 11: 722482.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences