Safety of Gynecomastia Procedures in Combination with Helium-Based Plasma Technology: A Retrospective Review
Paul Ruff1*, Rikesh Parikh2, Arian Mowlavi3, Edward Zimmerman4 and Jonathan Lebowitz5
1Department of Plastic Surgery, West End Plastic Surgery, Washington, USA
2Department of Plastic Surgeon, Bellevue, USA
3Plastic Surgeon, the Cosmetic Plastic Surgery Institute, Newport Beach, USA
4Cosmetic Surgeon, Aesthetic Revolution Las Vegas, Las Vegas, USA
5Plastic Surgeon, Lebowitz Plastic Surgery, Huntington, USA
- *Corresponding Author:
- Paul Ruff
Department of Plastic Surgery,
West End Plastic Surgery, Washington,
USA,
E-mail: drruff@westendplasticsurger.com
Received date: September 6, 2022, Manuscript No. IPARS-22-14486; Editor Assigned date: September 9, 2022, PreQC No. IPARS-22-14486 (PQ); Reviewed date: September 15, 2022, QC No. IPARS-22-14486; Revised date: September 25, 2022, Manuscript No. IPARS-22-14486 (R); Published date: October 3, 2022, DOI: 10.4172/2472-1905.8.6.152
Citation: Ruff P, Parikh R, Mowlavi A, Zimmerman E and Lebowitz J (2022) Safety of Gynecomastia Procedures in Combination with Helium-Based Plasma Technology: A Retrospective Review. J Aesthet Reconstr Surg Vol.8 No.6: 152.
Abstract
Background: The goal of treating gynecomastia is to restore a normal chest wall contour with few complications and minimal scar formation. The helium-based plasma technology (Renuvion; Apyx medical), that was used in combination with the gynecomastia procedures in this retrospective study, received 510(k) clearance on July 18, 2022 from the U.S. Food and Drug Administrations (FDA) for the use of the Renuvion APR hand piece for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax skin in the neck and submental region.
Objectives: To evaluate safety and procedure data for the treatment of gynecomastia in which a helium plasma device was used to coagulate and contract soft tissue.
Methods: A retrospective chart review was conducted in five medical centers to identify eligible subjects, adverse events, procedures, and follow-up time. Eligible subjects were males ≥21 years of age whose treatment included liposuction and the use of a helium-based plasma device (Renuvion; Apyx medical). Most subjects also underwent liposuction in other body areas (abdomen, thighs, arms, chin, neck, flanks, back, scapular rolls, mons pubis, buttocks, infra-axillary region) during the same treatment session. Demographic data, procedure details, and treatment settings were collected. Treatment-related complications and Adverse Events (AEs) were also recorded and evaluated.
Results: No serious adverse events were observed. Devicerelated adverse events were noted in 4 out of 84 subjects (5%) and totaled 6 events; 3 seroma and 3 hematoma events. All subjects with adverse events were treated with a co0mbination of devices (liposuction and Renuvion) during the gynecomastia procedure. All adverse events resolved.
Conclusions: The data collected supports the use of the helium plasma device as safe when used in combination with gynecomastia correction procedures.
Keywords
Coagulation; Contraction; Soft tissue; Gynecomastia; Skin laxity
Introduction
Gynecomastia is characterized by enlargement of the male breast caused by proliferation of tissue. The condition may be unilateral or bilateral, symmetric or asymmetric [1]. Affected patients may experience depression, anxiety, low self-esteem, and social phobia [2].
Gynecomastia has a reported incidence in up to 65% of males [3]. The condition may be due to excess of estrogens, androgen deficiency (hypogonadism), hormone resistance, or an altered ratio of estrogens to androgens. Diagnosis can be made by ultrasound, mammography, or both [4].
The goal of surgically treating gynecomastia is to correct the condition, while improving symmetry and chest contour, with few complications and minimal scar formation [5]. The author’s approach to this treatment generally involves tumescent infiltration and ultrasound-assisted liposuction to breakdown fat cells, detach them from deeper tissues, and remove them [6]. If there is a noticeable surplus of skin, the remaining tissue may be excised by periareolar incision. If not excised, excess skin settles to some degree, depending on skin quality [7].
In the author’s experience, surgical removal of excess skin is the conventional approach to treating skin laxity, but it has also been associated with increased risk of complications, a longer recovery period, and increased potential for scarring as opposed to the minimally invasive procedures they perform. These disadvantages have led to a shift in the industries focus on developing less invasive procedures to treat skin laxity.
Since the 1990s, Radiofrequency (RF), laser, and plasma devices have been used to heat and coagulate sub-dermal tissue leading to collagen contraction, thus improving the appearance of facial wrinkles and rhytides [8-13]. Due to the results, the use of thermal-induced collagen/tissue contraction has been expanded to minimally invasive procedures such as Laser- Assisted Lipolysis (LAL) and Radiofrequency-Assisted Lipolysis (RFAL). LAL and RFAL devices have combined the removal of subcutaneous fat with soft tissue heating to target the skin laxity that often results from fat volume removal.
A helium-based plasma device (Renuvion®, Apyx™ medical corporation, clear-water, FL) has been cleared by the US Food and Drug Administration (FDA) and is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation, and ablation of soft tissue during open surgical procedures; for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region; and to be used with compatible electrosurgical generators owned by Apyx medical (specifically BVX-200H, BVX-200P, APYX-200H, APYX-200P, APYXRS3, and APYX-JS3)(J220970). The helium-based plasma device consists of a hand piece, an electrosurgical generator, a supply of helium gas, and accompanying accessories. The generator delivers RF energy to the hand piece and energizes an electrode. When helium gas passes over the energized electrode, helium plasma is produced and the resulting heat is applied to the target tissue, leading to collagen/tissue contraction and correction of skin laxity.
The objective of the present study was to evaluate the safety of this helium plasma device when used for sub-dermal coagulation (and subsequent collagen/tissue contraction) in the treatment of gynecomastia by liposuction.
Methods
A retrospective chart review was conducted with a waiver of consent granted by Sterling IRB (Atlanta, GA). The medical charts or electronic medical records of five medical centers were reviewed to identify eligible subjects and record adverse events, procedure data, and last follow-up time point. IRB approval was obtained through Sterling Institutional Review Board on March 11, 2020. Eligible subjects were males ≥21 years of age who underwent a gynecomastia procedure in which a helium plasma device was used as a tool for sub-dermal coagulation after liposuction. Subjects not meeting these criteria were excluded.
One of the study sites received the updated version of the Apyx generator (APYX-RS3) in early 2020. This generator delivers the same standard mono-polar and bipolar electrosurgical energy in addition to helium gas plasma energy to soft tissue. The helium-based plasma portion of the generator contains a feature that permits the user to quantify the amount of energy delivered to tissue during treatments (kJ). The study site began to capture the amount of energy applied during the gynecomastia correction procedures and this data was available for 15 subjects in this retrospective chart review.
All subjects were seen per site standard of care for posttreatment follow-up at the discretion of the site physicians.
Data from charts were analyzed by either the Wilcoxon-Mann- Whitney test or Pearson’s chi-squared test using P<0.05 as the cutoff for significance. These non-parametric tests were utilized due to the data having either small whole numbers with a short range, not normally distributed as shown by the Shapiro-Wilk test, or both.
Results
Charts of males (n=84) aged 21 to 65 years (mean 40 years) and BMI 23.0 to 37.8 (mean 29.5) were reviewed for this study. All subjects were treated with liposuction and a helium-based plasma device. Many subjects (n=69, in addition to gynecomastia correction, had additional body areas (abdomen, thighs, arms, chin, neck, hips, back, scapular rolls, mons pubis, buttocks, infra-axillary region) treated during the same procedure session.
Liposuction was accomplished with an Ultrasound-Assisted Liposuction (UAL) device (VASER™,© 2018 Solta medical, bausch health companies Inc., Laval, Quebec, Canada) in 16 treatment areas (n=69), a Suction-Assisted Liposuction (SAL) device in 7 treatment areas (n=7), or Power-Assisted Liposuction (PAL) device in 1 treatment area (n=8). The infiltrate mixture was 50 ml of 2% lidocaine and 0.5 ml of epinephrine (1 mg/ml) distributed across both sides of the chest. Undermining was performed by ultra-sound assisted device with power (n=69) or by cannula without power (n=15). Excision of glandular tissue for gynecomastia correction was performed in 70% (n=59) of subjects.
Across all procedure variations reviewed in this study, the average helium-based plasma device settings were 75% power, 3 Liters Per Minute (LPM), and 5 passes. Of the 15 subjects treated with the helium plasma device where “amount of energy applied” data was available, the average total bilateral energy used was 10.1 kJ (range: 8-20 kJ. No adverse events were reported for these subjects where the amount of energy applied was available.
Subjects were seen per site standard of care for postoperative follow-up. The mean patient follow-up postoperatively was 1.5 months (range: 1 day-37.5 months) postprocedure.
No serious adverse events were observed. A total of 5 types of adverse events (swelling, bruising, hematoma, seroma, diminished spOâ??) were reported among 6 subjects. There were 6 device-related adverse events noted in 3 body areas (Hip (n=2), Abdomen (n=1), and Chest (n=3)) in 5% of subjects (n=4) (Table 1).
| Subject | Adverse Event | Area | Severity | Liposuction Technique | Related to Device | Treatment |
|---|---|---|---|---|---|---|
| 01-002 | Swelling | Bilateral flank | Mild | UAL | Unlikely | None |
| 01-006 | Bruising | Abdomen | Mild | UAL | Unrelated | Ice Packs |
| 01-006 | Hematoma | Left chest | Moderate | UAL | Possibly | Aspirated Serous Fluid |
| 01-008 | Seroma | Right hip | Mild | UAL | Possibly | Aspirated Serous Fluid |
| 01-008 | Seroma | Right lower quadrant | Mild | UAL | Possibly | Aspirated Serous Fluid |
| 01-009 | Seroma | Right side of chest | Mild | UAL | Possibly | Aspirated Serous Fluid |
| 01-009 | Hematoma | Right chest wall | Severe | UAL | Possibly | Aspirated Serous Fluid |
| 02-001 | Hematoma | Unilateral chest | Mild | PAL | Possibly | Washed out in OR |
| 03-001 | Diminished spO2 | Airway | Mild | SAL | Unrelated | Intubation |
Table 1: Adverse events.
spO2 = Oxygen saturation level.
UAL = Ultrasound-assisted liposuction.
PAL = Power-assisted liposuction.
SAL = Suction-assisted liposuction
Of the device-related adverse events, 3 were seromas; 2 were in the hip and 1 was in the abdomen. All areas where a seroma occurred had been treated with ultrasound-assisted liposuction in conjunction with the helium-based plasma device. There were no reports of seroma adverse events in subjects treated with PAL or SAL in conjunction with the helium-based plasma device.
Two (2) of the hematoma events occurred in areas treated with UAL in conjunction with the helium-based plasma device and 1 occurred in an area treated with PAL in conjunction with the helium-based plasma device. As multiple devices were used to treat these subjects, it is not possible to identify which device or combination of devices contributed to the adverse events. Three (3) events unrelated/unlikely related to the device(s) were also reported. All adverse events resolved.
Subjects with and without device-related adverse events were compared for potential association with demographic, procedural, and other variables (Table 2).
| Variable | Adverse events | No adverse events | P value |
|---|---|---|---|
| Demographic | Median (range) | ||
| Age | 53.5 (38-61) | 37.0 (21-65) | 0.0329 |
| BMI | 31.6 (28-36) | 29.5 (23-38) | 0.2676 |
| Procedural | |||
| Helium plasma device | |||
| Power | 80.0 (40-85) | 80.0 (20-85) | 0.7362 |
| Helium flow (L/min) | 3.0 (2.0-4.0) | 2.5 (1.5-4.0) | 0.0993 |
| No. of passes | 4.0 (2.0-6.0) | 5.0 (2.0-6.0) | 0.3895 |
| Follow-up (days) | 45.0 (30-248) | 7.5 (1-485) | 0.0832 |
| Other | Proportions (%) | ||
| Lipo technique UAL SAL PAL |
3/4 (75), 1/7 (25), 0/4 (0) |
65/78 (83), 13/78 (17), 0/78 (0) |
0.4013 |
| Tissue extraction Yes No |
3/4 (75), 1/4 (25) | 55/78 (71), 23/78 (29) |
0.8475 |
Table 2: Demographic and procedural variables potentially associated with device-related adverse events (seroma, hematoma).
Range = Maximum minus minimum.
The median age of AE subjects was significantly higher than that of subjects without AEs (p=0.0329). Differences were not significant for BMI, helium plasma procedural variables, followup time, liposuction technique, and whether or not subjects had tissue extraction. Statistical analysis was limited, due to the small number of subjects (n=4) that experienced device-related adverse events. Other adverse events experienced by subjects were bruising (n=1, 1%), swelling (n=1, 1%), diminished oxygen saturation (n=1, 1%).
Discussion
The device-related adverse events in this study are associated with age and liposuction technique. The most frequently occurring adverse event was seroma which was observed only in patients treated with both ultrasound-assisted and heliumbased plasma device procedures. In the authors’ experience, seroma development is increased with the additional delivery of ultrasonic energy.
Seroma is a known and expected risk of ultrasound-assisted liposuction and has been associated with these procedures [14-20]. Reported rates of seroma range from 0 to 29.9% in studies with up to 1,772 patients. In the present study, seroma was noted in 2% of 84 patients, which compares favorably with the overall seroma rate of 6.9% for UAL alone established by combining the data for 3,968 total patients from all of the aforementioned studies. Recently, a study evaluated the Adverse Events (AEs) of procedures in which a Helium-Based Plasma Technology (HPT) was used with and without Ultrasound-Assisted Liposuction (UAL), seroma was the most frequently occurring AE in patients treated with both UAL and HPT, but was not observed in patients whose body areas were treated solely with HPT. This absence of seroma AE in patients treated solely with HPT could be due to the capabilities of the device; rapid cooling that occurs in the tissue surrounding the treatment site and the targeted soft tissue coagulation/ contraction occurrence without heating the full thickness of the dermis [17].
Increased energy delivered to the tissue could cause increased seroma rates. In the aforementioned recent study patients treated with a UAL power setting greater than 70%were 7.63 times more likely to have an AE than those treated within 40%-70% power setting range [17]. This is shown in the data of this study as well i.e. higher ultrasound-assisted liposuction power settings resulted in higher incidence of seroma. Therefore, users should be careful when “stacking” multiple energy sources in a procedure. Consider lower power settings or fewer passes when planning to use multiple energy sources on the same tissue.
As stated earlier, the goal of treating gynecomastia is to correct the condition with few complications and minimal scar formation [5]. Excess skin after surgery may be noticeable, in which case excision is indicated [7]. In 1979, Davidson described a technique for excising skin in gynecomastia operations in which he used a concentric “circle” design to remove a calculated amount in the vertical and the horizontal directions [21,22]. The result was a circular scar around the areola [20]. This technique was recently used to remove excess skin in 16 of 50 patients with gynecomastia. A hematoma developed in 1 Davidson patient and a hypertrophic scar developed in another. In the Longheu study, Davidson patients required more frequent use of drains and the average duration of stay in the hospital was longer than in non-Davidson patients [1].
In the present study, the authors used the helium-based plasma device, rather than the Davidson technique, to correct skin laxity after the gynecomastia correction procedure. Following the liposuction and consequent removal of fat and glandular tissue, the helium-based plasma device was inserted through the same incisions used for the liposuction portion of the procedure, thus minimizing downtime and scarring. The device allows for contraction of the skin and fibrous septa, especially in patients with skin of little elasticity preoperatively. Anecdotally, the authors report a range of 10% to 30% more contraction than without the use of the heliumbased plasma device. In the authors’ opinion, the device can provide predictable and reproducible contraction without applying large amounts of heat to the tissue.
Due to the minimally invasive nature of the gynecomastia procedures performed in this retrospective study, patients were able to present for their procedure and would return home post-operatively, as is typical for gynecomastia procedures. In the author’s practices, patients are typically seen for one or two follow-up visits within the first week following their procedure. After completion of these routine follow-up appointments, extended follow-up was not included as a standard of practice or necessary unless there was a persistent issue. In these circumstances, these visits were captured, and AEs were documented.
In addition to inconsistent follow-up timing among subjects, this study’s retrospective design limited the data available for retrospective collection. Objective and subjective measures of patient’s outcomes and satisfaction were not recorded.
Although this study is limited by its retrospective design and data availability, the results show that use of the helium-based plasma device in the treatment of gynecomastia by liposuction is safe, even when other body areas are treated in the same session and may reduce the risk of adverse events such as posttreatment scars.
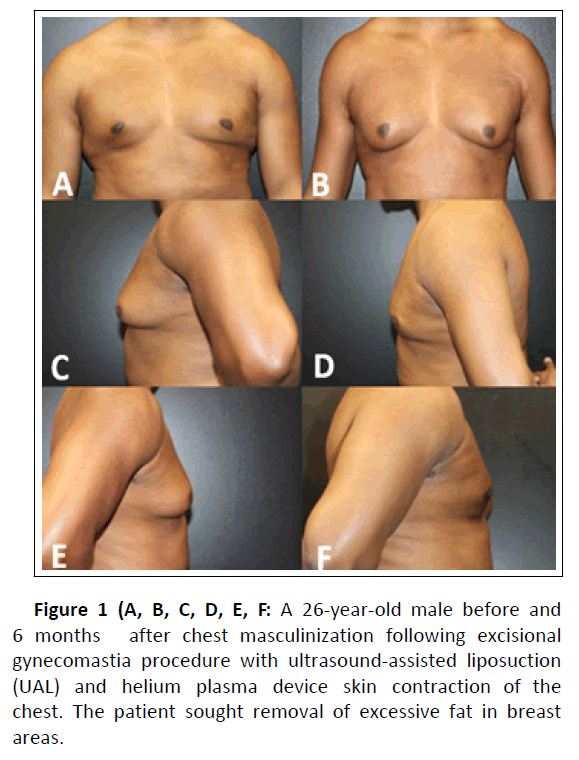
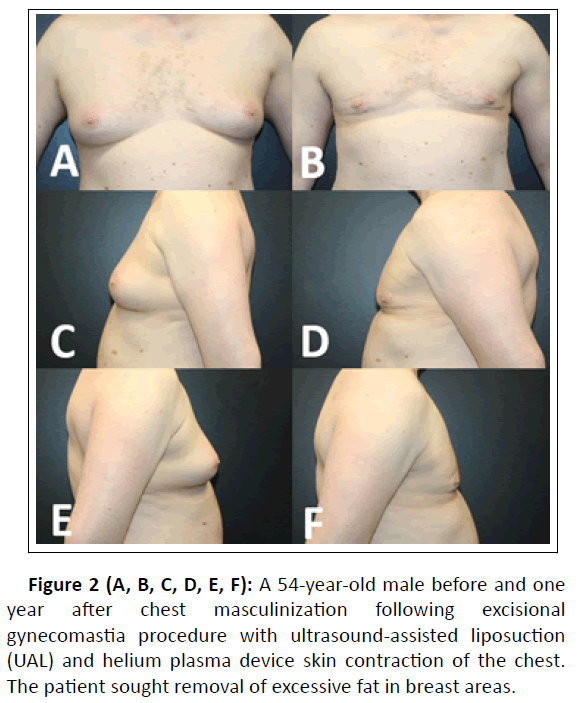
Clinical examples of patient outcomes can be found in Figures 1, 2, 3, and 4.
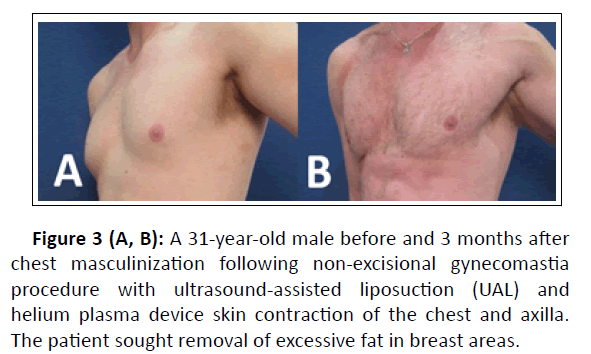
Figure 3 (A, B): A 31-year-old male before and 3 months after chest masculinization following non-excisional gynecomastia procedure with ultrasound-assisted liposuction (UAL) and helium plasma device skin contraction of the chest and axilla. The patient sought removal of excessive fat in breast areas.
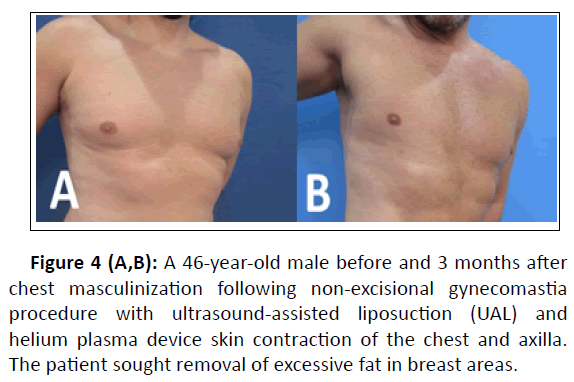
Figure 4 (A,B): A 46-year-old male before and 3 months after chest masculinization following non-excisional gynecomastia procedure with ultrasound-assisted liposuction (UAL) and helium plasma device skin contraction of the chest and axilla. The patient sought removal of excessive fat in breast areas.
Conclusions
The data collected from a review of 84 male patient charts from five sites supports the use of a helium plasma device as safe when used in combination with liposuction in gynecomastia correction procedures.
Disclosures
The study was funded by Apyx Medical Corporation. Physicians received site payments for their participation in this retrospective study. Dr. Zimmerman and Dr. Ruff are medical advisory board members, consultants, and clinical research investigators for Apyx medical and receive compensation in the form of Apyx stock options and hourly compensation. Dr. Mowlavi and Dr. Lebowitz are also consultants and clinical research investigators for Apyx medical. Dr. Parikh has no financial relationship with Apyx medical outside of study funding.
References
- Longheu A, Medas F, Corrias F, Farris S, Tatti A et al. (2016) Surgical management of gynecomastia: Experience of a general surgery center. G Chir. 37: 150-154.
[Crossref ], [Google Scholar], [Indexed]
- Akhtar A, Eitezaz F, Rashid M, Khan I, Malik SA (2019) Liposuction in Gynecomastia: An Assessment of the Suction-assisted Arthroscopic Shaver Versus Open Disc Excision Techniques. Cureus. 11: e5897.
[Crossref ], [Google Scholar], [Indexed]
- Holzmer SW, Lewis PG, Landau MJ, Hill ME (2020) Surgical Management of Gynecomastia: A Comprehensive Review of the Literature. Plast Reconstr Surg Glob Open. 8: e3161.
[Crossref ], [Google Scholar], [Indexed]
- Costanzo PR, Pacenza NA, Aszpis SM, Suárez SM, Pragier UM et al (2018) Clinical and Etiological Aspects of Gynecomastia in Adult Males: A Multicenter Study. Biomed Res Int. 2018: 8364824.
[Crossref ], [Google Scholar], [Indexed]
- Taheri AR, Farahvash MR, Rathi HR, Ghanbarzadeh K, Faridniya B (2016) The Satisfaction Rate among Patients and Surgeons after Periareolar Surgical Approach to Gynecomastia along with Liposuction. World J Plast Surg. 5: 287-292.
[Crossref ], [Google Scholar], [Indexed]
- Caridi RC (2018) Defining the Aesthetic Units of the Male Chest and How They Relate to Gynecomastia Based on 635 Patients. Plast Reconstr Surg. 142: 904-907.
- Arvind A, Khan MA, Srinivasan K, Roberts J (2014) Gynecomastia correction: A review of our experience. Indian J Plast Surg. 47: 56-60.
[Crossref ], [Google Scholar], [Indexed]
- Fatemi A, Weiss MA, Weiss RA (2002) Short-term histologic effects of nonablative resurfacing: results with a dynamically cooled millisecond-domain 1320nm Nd:YAG laser. Dermatol Surg 28: 172-176
[Crossref ], [Google Scholar], [Indexed]
- Hsu T, Kaminer M (2003) The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg 22: 115-123.
[Crossref ], [Google Scholar], [Indexed]
- Alster TS, Doshi SN, Hopping SB (2004) Combination surgical lifting with ablative laser skin resurfacing of facial skin: a retrospective analysis. Dermatol Surg 30: 1191-1195.
[Crossref ], [Google Scholar], [Indexed]
- Zelickson B, Kist D, Bernstein E, Brown DB, Ksenzenko S et al (2004) Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: A pilot study. Arch Dermatol 140:204-209
[Crossref ], [Google Scholar], [Indexed]
- Doshi SN, Alster TS (2005) Combination radiofrequency and diode laser for treatment of facial rhytides and skin laxity. Cosmet Laser Ther 7: 11-15.
[Crossref ], [Google Scholar], [Indexed]
- Mayoral FA (2007) Skin tightening with a combined unipolar and bipolar radiofrequency device. J Drugs Dermatol 6: 212-215.
[Google Scholar], [Indexed]
- Jewell ML, Fodor PB, de Souza Pinto EB, Al Shammari MA (2002)Clinical application of VASER-assisted lipoplasty: A pilot clinical study. Aesthetic Surgery Journal. 22 :131-46.
[Crossref ], [Google Scholar], [Indexed]
- Hoyos AE, Millard JA (2007) VASER-assisted high-definition liposculpture. Aesthet Surg J. 27: 594-604.
[Crossref ], [Google Scholar], [Indexed]
- Roustaei N, Lari SJ, Chalian M, Chalian H, Bakhshandeh H (2009) Safety of ultrasound-assisted liposuction: a survey of 660 operations. Aesthetic Plastic Surgery 33: 213-8.
[Crossref ], [Google Scholar], [Indexed]
- VP-1910 Seroma Title: Clinical Study Report. 14-17.
- Hoyos A, Perez ME, Guarin DE, Montenegro A (2018) A report of 736 high-definition lipoabdominoplasties performed in conjunction with circumferential VASER liposuction. Plast Reconstr Surg 142: 662-75.
[Crossref ], [Google Scholar], [Indexed]
- Danilla S, Babaitis RA, Jara RP, Quispe DA, Andrades PR et al.,(2020) High definition liposculpture: What are the complications and how to manage them? Aesthetic Plast Surg 44: 411-8.
[Crossref ], [Google Scholar], [Indexed]
- Hoyos AE, Perez ME, Domínguez-Millan R (2020) Variable Sculpting in Dynamic Definition Body Contouring: Procedure Selection and Management Algorithm. Aesthet Surg J 41: 318-332.
[Crossref ], [Google Scholar], [Indexed]
- Ruff P, Vanek P, Nykiel M (2022) Adverse event of soft tissue coagulation using a helium-based plasma technology alone and in combination with ultrasound-assisted liposuction. Aesthet Surg J
[Crossref ], [Google Scholar], [Indexed]
- Davidson BA (1979) Concentric circle operation for massive gynecomastia to excise the redundant skin. Plast Reconstr Surg 63: 350-4
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences