Association of Microbial Growth on Silicone Breast Implants with Capsular Contracture: A Systematic Review
Melissa Agnello, Payal Shah, Jonathan Tucci, Stefanie Bodison, Maxwell Johnson, Daniel Gardner and Alex K Wong
DOI10.4172/2472-1905.100007
Division of Plastic and Reconstructive Surgery, Keck School of Medicine of USC, USA
- *Corresponding Author:
- Alex K Wong
Assistant Professor of Surgery, Director
Microsurgery Fellowship, Division of Plastic and Reconstructive Surgery
Keck School of Medicine of USC, USA
Tel: (323) 442-7920
E-mail: Alex.Wong@med.usc.edu
Received date: November 17, 2015; Accepted date: November 20, 2015; Published date: November 30, 2015
Citation: Agnello M, Shah P, Bodison JTS, et al. Association of Microbial Growth on Silicone Breast Implants with Capsular Contracture: A Systematic Review. J Aesthet Reconstr Surg. 2016, 1:7. doi: 10.4172/2472-1905.10007
Abstract
Background: Capsular contracture is the most common complication following breast implant surgery. Previous evidence suggests that the presence of microbial biofilms on and surrounding the implant may contribute to the development of contracture.
Objective: Our goal was to systematically review the literature and summarize the evidence to date regarding the identification of biofilm-forming bacteria on breast implants and the association with capsular contracture. Furthermore, we aimed to describe specific microbial species associated with capsular contracture and assess if species correlates with contracture severity.
Exposure and outcomes of included studies: 10 studies were included in the present review, consisting of cross sectional studies of patients undergoing breast implant removal, as well as experimental animal models. All studies assessed for the presence of microbial growth and subsequently assessed capsular contracture using the Baker’s scale.
Conclusion: Results of all studies support the hypothesis that the presence of microbial biofilm is a major contributor to capsular contracture, with the primary causal species being Staphylococcus epidermidis and Propionibacterium acne, part of the normal breast flora. Better powered and better controlled studies are needed to further investigate this phenomenon in order to inform surgeons on how to mitigate the risk of capsular contracture for patients.
Introduction
According to the American Society of Plastic Surgeons National Clearinghouse of Plastic Surgery Procedural Statistics, 296,203 breast augmentation procedures and 93,083 breast reconstruction procedures were performed in the United States in 2010. Approximately half of these procedures included the use of silicone breast prosthesis [1]. While silicone breast implants have been deemed safe, local complications can arise. Although rare, the most common immediate post-operative complications of breast implant surgery include hematoma, seroma, wound infection, and malposition/asymmetry.
The most frequent late complication is capsular contracture which is characterized by an abnormal thickening of the collagen fiber capsular tissue that forms around all implants and leads to breast pain and/or malposition of the breast implant. Capsular contracture occurs in 1.3 to 30% of patients depending on the case series [2,3]. Mentor Corporation reported the 3-year cumulative incidence to be 5.3% for augmentation, 11.8% for revisionaugmentation, 9.1% for reconstruction, and 10% for revisionreconstruction [1]. While the mechanism of capsular contracture involves dysregulated inflammation of the surrounding tissue following implantation the etiology of this disease process is not fully understood [4]. Nevertheless, several variables have been linked to increased incidence of capsular contracture including smooth implant shell (as opposed to textured) [5,6] and submuscular (versus sub-glandular) implant positioning [7].
Microbial colonization, specifically with biofilm-forming bacteria, has been associated with the occurrence of capsular contracture in patients [8,9]. Specifically, the skin microbe Staphylococcus epidermidis has been implicated as the infection-causing agent [9] in some cases. Although it is highly plausible that microbial contamination can induce maladaptive inflammation that would lead to capsular contracture, there is controversy regarding the linkage between this risk factor and the disease process. Thus, our objective is to systematically review the literature and summarize the evidence to date regarding the identification of biofilm-forming bacteria on breast implants and the association with capsular contracture. Furthermore, we aim to describe specific microbial species associated with capsular contracture and assess if species correlates with contracture severity.
Method
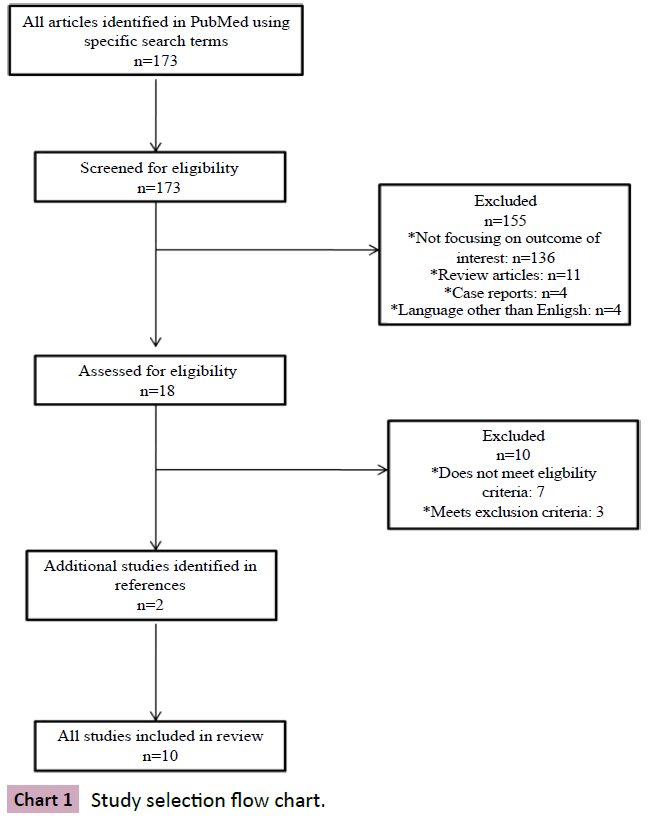
In order to accurately identify and assess the published information regarding microbial growth on breast implants and the occurrence of capsular contracture, we followed a pre-defined protocol for selecting studies. The following search paradigm was used in PubMed: “(silicone implant OR breast augmentation OR breast reconstruction) AND (capsular contracture) AND (biofilm OR infection).” All articles published after 1966 were screened for eligibility by reading the abstracts. The references of each included study were also screened for possible inclusion.
Inclusion criteria
The following types of studies were considered for inclusion: prospective and retrospective observational cohort studies, as well as experimental studies involving animal models. Types of participants included patients that have undergone breast augmentation surgery or breast reconstruction surgery. Studies using murine, rabbit, or porcine animal models were also considered. Only studies specifically containing data on the occurrence of capsular contracture as the primary outcome in study participants were included. For the exposure, only studies that specifically analyzed the presence of microbial growth at the time of surgery were considered.
Exclusion criteria
Studies that did not include original data, such as review papers, were excluded. We also excluded unpublished data, papers published before 1966, and papers published in a language other than English.
Data extraction
Relevant data were extracted from the studies selected for the review and are presented in Chart 1. Essential information collected from each study included: participant information, exposure (microbial growth, presence, and identification), means of assessing the exposure, outcome assessment (capsular contracture) and severity of the outcome.
Quality assessment
Quality was assessed based on the risk of bias present in each study. Bias for each individual study was assessed using a previously published assessment tool [10] and based on the criteria; each study was placed in a bias risk level tier ranging from 1-3, with Tier 3 having the highest risk of bias.
Results
Study selection
The selection process is detailed in Chart 1. Briefly, the search terms produced 173 results, which, after initial screening, were narrowed down to 18. After critical reading, an additional 10 studies were excluded. Two additional studies were identified and added from the references of the included studies. In total, 10 studies were included in the present review.
Characteristics and results of included studies
The details and extracted data of each study are described in Chart 1. Most of the included studies were prospective or crosssectional cohort studies, focusing on patients undergoing either reconstructive or aesthetic breast surgery. Three studies utilized animal models: one porcine [11] one guinea pig [12], and one rabbit [13]. In these studies the authors inserted miniature gelfilled implants into the animals. Prior to insertion, the implant pockets were either left sterile, or inoculated. In the porcine and rabbit studies, the pockets were inoculated with a strain of the common human skin flora Staphylococcus epidermidis that had been isolated from a patient with capsular contracture. In both studies, the implants inoculated with S. epidermidis more frequently developed more severe (Grade III and IV) capsular contracture than the sterile controls (100% vs. 0% in the rabbit study, and 78% vs. 47% in the porcine model). In the guinea pig model, the sites were inoculated with Staphylococcus aureus. The results of this study suggested that capsular contracture was equally likely to occur in sterile and inoculated implantations; however, the presence of the microbe accelerated and worsened the effect.
Studies on patients were observational and fell into two types: prospective, in which patients were followed from the time of implantation, and cross-sectional. The 6 cross-sectional studies [8,9,14-17] analyzed implants at the time of removal due to complications (capsular contracture or otherwise). In general, these studies enrolled participants at the time of removal of one or both of their implants, and analyzed both the outcome (contracture) and the exposure (microbial growth) at the same time point. All 6 of these studies found a positive correlation between the diagnosis of capsular contracture and the presence of microbes. In two of these studies, [8,16] only implants removed due to contracture were analyzed, and the authors found a significant association of microbial growth with increasing severity of contracture (Baker’s grade III and IV compared to grade I and II). A similar finding was presented by Pajkos et al [9], in which the presence of Staphylococcus epidermidis was associated with Baker III/IV capsular contracture, and not Baker I/II.
One of the included studies followed a cohort of patients starting from the time of implantation and for the subsequent year [18]. Although the numbers were small, the results indicated that the patients who developed contracture were more likely to have had a positive culture during the time of implantation (primarily Staphylococcus epidermidis and Propionibacterium acnes).
In general, both the observational and experimental studies provide strong evidence that the presence of bacteria on the implant or inside the implant pocket is a major driving factor for the occurrence of capsular contracture. The major species identified across all the studies are Staphylococcus epidermidis and Propionibacterium acnes, although other species were identified as well. None of the studies correlated specific microbial species with severity of capsular contracture.
Discussion
Capsular contracture of the breast tissue accounts for almost 75% of the complications seen following breast augmentation or reconstructive surgery [19]. In the United States alone, 45,000 individuals are affected per year, and 15-30% of patients that have undergone breast augmentation or reconstruction suffer from this complication [20]. Capsular contracture results from the inflammation of the surrounding tissue following implantation; however, the etiology is not fully understood [4]. The presence of biofilm-forming bacteria has been associated with the occurrence of capsular contracture in patients [8,9]. In the current systematic review, we aimed to gather the published evidence regarding the association of microbial growth with capsular contracture.
The present review included 10 studies published from 1988- 2013. The evidence in the literature overwhelmingly supports the hypothesis that microbial growth is a driving factor in the development of capsular contracture. Both cross-sectional studies and experimental studies using animal models show that the presence of microbial growth, especially biolfilms, is strongly correlated with contracture. Biologically, biofilms are complex networks of bacteria that allow it to adhere to surfaces. Prostheses, such as breast implants, are a common location of biofilm attachment, where it can lead to recurrent infections. Biofilms are antibiotic resistant, making them difficult to eradicate. Furthermore, continuous microbial presence on the breast implant may induce low-grade inflammation in the surrounding tissue, eventually leading to contracture [21]. The majority of the results identified the common skin flora S. epidermidis and P. acnes as the likely culprits. These species have been identified as normal components of the breast skin and surrounding tissue [22]. However, no association with a single species and the degree of contracture was investigated in any of the included studies.
It would be interesting to investigate if contamination with nonnormal flora leads to more severe outcomes.
Because capsular contracture is such a common complication that may cause a variety of problems for patients, reviews of the literature are essential to guide surgeons in how best to prevent complications. For example, it is apparent that normal skin flora of the breast is a main source of contamination of the implant; therefore, surgeons may be inclined to wash the area with an antibiotic solution or cover certain areas to prevent contamination. One group suggests that the use of a nipple shield can prevent contamination from the nipple [23]. A randomized controlled trial [24] examined the preventative effects of washing the implant area with various antibiotic solutions before implantation, and found that this was a highly effective method for preventing contracture. However, the scarcity of such studies furthers the need for evidence from many smaller observational studies to be brought together, as in the present review.
There are some limitations of this review; namely, few observational studies have been published on a large cohort. The included studies generally describe relatively small sample sizes, and the selection of patients seems to be out of convenience.
For example, many of the studies included in this review used a patient population that was undergoing removal of their breast implants due to complications, such as capsular contracture. Recruiting participants in this way may introduce bias due to over-selecting from the case population.
Because the studies included in this review span four decades, the methods for detecting the presence of microbes is different in the older studies compared to the newer studies. The older studies relied on standard microbiological culturing techniques, which may have been missed organisms that are scarce or difficult to culture. More recent studies utilized the sonication method [14,16,17] in which the entire implant is dissolved with sonication and cultured. As microbiological techniques evolve, the detection of microbial species will become more sensitive and additional investigations into the association of complications related to these microbes will be warranted. Recently, the Human Microbiome Project has identified the organisms present on a variety of body sites in healthy individuals using high-throughput sequencing technology. Applying new technology to the investigations into the etiology of capsular contracture may help to guide surgeons on how best to prevent this complication.
References
- FDA Update on the Safety of Silicone Gel-Filled Breast Implants (2011) In: Food and Drug Administration CfDaRH, USA.
- Araco A, Gravante G, Araco F, Delogu D, Cervelli V, et al. (2007) A retrospective analysis of 3,000 primary aesthetic breast augmentations: postoperative complications and associated factors. Aesthetic PlastSurg31:532-539.
- Handel N, Cordray T, Gutierrez J, Jensen JA (2006)A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg117:757-767.
- Adams WP (2009) Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg36:119-126.
- Barnsley GP, Sigurdson LJ, Barnsley SE (2006) Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg117:2182-2190.
- Wong CH, Samuel M, Tan BK, Song C (2006) Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg118:1224-1236.
- Gutowski KA, Mesna GT, Cunningham BL (1997) Saline-filled breast implants: a Plastic Surgery Educational Foundation multicenter outcomes study. Plast Reconstr Surg100:1019-1027.
- Schreml S, Heine N, Eisenmann-Klein M, Prantl L (2007) Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammaplasty. Ann PlastSurg59:126-130.
- Pajkos A, Deva AK, Vickery K, Cope C, Chang L, et al. (2003) Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg111:1605-1611.
- Draft Protocol for Systematic Review to Evaluate TheEvidence For An Association Between Bisphenol A (BPA) Exposure and Obesity (2013) In: Office of Health Assessment and Translation (OHAT) DotNTP, National Institute of Environmental Health Sciences, USA.
- Tamboto H, Vickery K, Deva AK (2010)Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty.Plast Reconstr Surg 126:835-842.
- Kossovsky N, Heggers JP, Parsons RW, Robson MC (1984) Acceleration of capsule formation around silicone implants by infection in a guinea pig model. Plast Reconstr Surg 73:91-98.
- Shah Z, Lehman JA, Tan J (1981) Does infection play a role in breast capsular contracture? Plast Reconstr Surg 68:34-42.
- Del Pozo JL, Tran NV, Petty PM (2009) Pilot study of association of bacteria on breast implants with capsular contracture. J Clin Microbiol 47:1333-1337.
- Dobke MK, Svahn JK, Vastine VL, Landon BN, Stein PC, et al. (1995) Characterization of microbial presence at the surface of silicone mammary implants. Ann Plast Surg 34:563-569.
- Rieger UM, Pierer G, Lüscher NJ, Trampuz A (2009) Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast Surg 33:404-408.
- Rieger UM, Mesina J, Kalbermatten DF (2013) Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg 100:768-774.
- Thornton JW, Argenta LC, McClatchey KD, Marks MW (1988) Studies on the endogenous flora of the human breast. Ann Plast Surg 20:39-42.
- Ersek RA (1991) Rate and incidence of capsular contracture: a comparison of smooth and textured silicone double-lumen breast prostheses. Plast Reconstr Surg 87:879-884.
- Cunningham B (2007) The Mentor Study on Contour Profile Gel Silicone MemoryGel Breast Implants. Plast Reconstr Surg120:33S-39S.
- Netscher DT (2004) Subclinical infection in breast capsules. Plast Reconstr Surg114:818-820.
- Bartsich S, AschermanJA, Whittier S, Yao CA, Rohde C (2011)The breast: a clean-contaminated surgical site. AesthetSurg J31:802-806.
- Wixtrom RN, Stutman RL, Burke RM, Mahoney AK, Codner MA (2012) Risk of breast implant bacterial contamination from endogenous breast flora, prevention with nipple shields, and implications for biofilm formation. AesthetSurg J32:956-963.
- Burkhardt BR, Dempsey PD, Schnur PL, Tofield JJ (1986) Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg77:919-932.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences