Evaluation of Breast Implants by Magnetic Resonance: A Pictorial Essay
Marcos Fernando de Lima Docema, Danbia Ariana de Andrade, Adolfo Previdelli Bolinelli, Pedro Srgio Brito Panizza, Ana Paula Piconi de Souza,Bernardo Nogueira Batista, Giovanni Guido Cerri, and Alfredo Carlos SD Barros
DOI10.4172/2472-1905.100006
1Magnetic Resonance Department, Hospital Sírio Libanês, São Paulo, SP, Brazil
2Mastology Department, Hospital Sírio Libanês, São Paulo, Brazil
3Plastic Surgery Department, Hospital Sírio Libanês, São Paulo, Brazil
4Imaging Diagnostics Department, Hospital Sírio Libanês, São Paulo, SP, Brazil
- *Corresponding Author:
- Marcos Fernando de Lima Docema
Radiologist, Magnetic Resonance Department
Hospital Sírio Libanês, São Paulo
SP, Brazil
Tel: +551131550571
E-mail: mdocema@gmail.com
Received date: October 06, 2015; Accepted date: November 13, 2015; Published date: November 21, 2015
Citation: Agnello M, Shah P, Bodison JTS, et al. Association of Microbial Growth on Silicone Breast Implants with Capsular Contracture: A Systematic Review. J Aesthet Reconstr Surg. 2016, 1:6. doi: 10.4172/2472-1905.10006
Introduction
Silicone-filled breast implants (SFBI) were introduced to clinical practice in 1962 [1]. Since then, they have become the preferred implants for either aesthetic or reconstructive breast surgery worldwide. In the United States, they have gained popularity among surgeons and patients since the Food and Drug Administration (FDA) moratorium on SFBI was raised in 2006 [1-5] versus their saline-filled counterparts, these implants feel softer to the touch and offer a more natural and stable shape. Furthermore, their safety has been demonstrated by a large number of studies that failed to prove any relation between SFBI and cancer or autoimmune diseases.
SFBI have evolved significantly over the last 50 years. The use of more resistant shells and higher cohesivity silicone gel reduced the rates of implant rupture. Nevertheless, “silent rupture” of a SFBI remains a point of major concern for patients and plastic surgeons. Capsular contracture is another relatively common complication of SFBI—especially in irradiated implant reconstructed breasts [6-9].
Either as a screening tool or as a complimentary evaluation for suspicious findings of a careful clinical examination, imaging methods are powerful allies of the plastic surgeon. Depending on convenience and availability, mammography, ultrasonography and magnetic resonance imaging (MRI) can be used to evaluate these implants. Due to the risk of silent ruptures, the FDA recommends that patients with SFBI-augmented breasts have an MRI three years postoperatively and every two years after that. Breast cancer survivors should also have MRIs during their oncological follow-ups. In the following article, we describe the MRI findings for breast implants as well as complications. It is a pictorial essay that is intended to help plastic surgeons interpret MRI images and have a better understanding of their reports.
Technical Considerations
MRI evaluation of breast implants requires a device with at least a 1.5 Tesla magnetic field, a dedicated coil to the evaluation of the breasts, and a study protocol with pulse sequences to acquire specific images that characterize the silicone content.
The exam should be performed with the patient in prone position with breasts pending inside the coil and acquisition of multiplanar images of the breasts weighted in T1 and T2. Water and fat saturation signals can be selectively imaged via different pulse sequences [10,11].
In our department, we recommend the use of intravenous paramagnetic contrast in all cases even when only implant evaluation is intended. This detects eventual malignancies that could only be seen in MRI. It also identifies inflammatory processes in breast parenchyma that could be associated with implant complications.
Types of Implants and Positioning
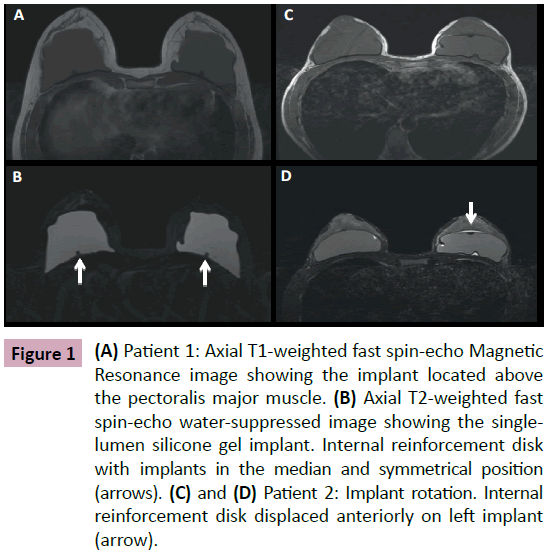
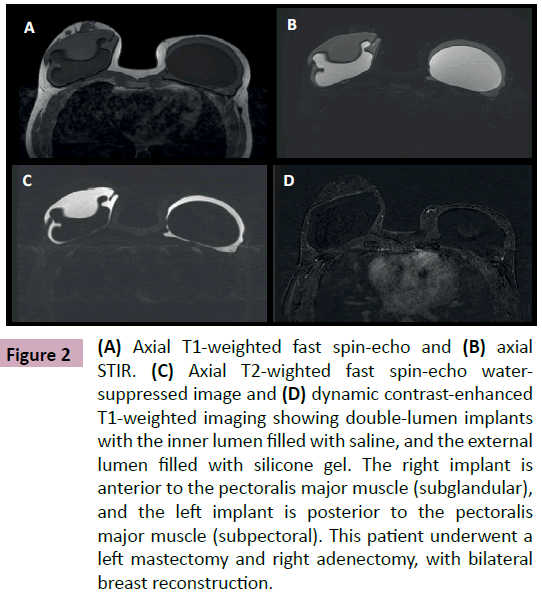
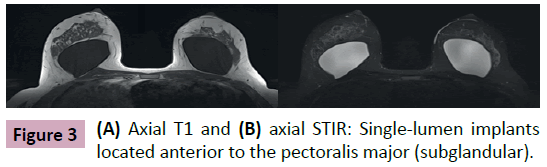
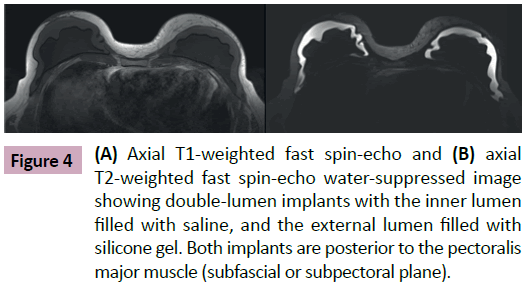
Breast implants consist of a silicone elastomer shell that can be filled with either saline solution or silicone gel (Figure 1). Despite the type of filling, these are single lumen implants. Expandable implants are a particular type of breast implant that is sometimes used in breast reconstruction. These are double lumen implants and in most cases their inner lumen is filled with saline while the external lumen is filled with silicone gel (Figure 2) [1-9]. They allow a gradual and controlled expansion of the reconstructed breast thru either an anterior, integrated valve or a distant valve placed in the subcutaneous tissue—this can be removed after expansion. Implants can be placed in a subglandular (Figure 3), subfascial or subpectoral plane (Figure 4).
Figure 1: (A) Patient 1: Axial T1-weighted fast spin-echo Magnetic Resonance image showing the implant located above the pectoralis major muscle. (B) Axial T2-weighted fast spin-echo water-suppressed image showing the singlelumen silicone gel implant. Internal reinforcement disk with implants in the median and symmetrical position (arrows). (C) and (D) Patient 2: Implant rotation. Internal reinforcement disk displaced anteriorly on left implant (arrow).
Figure 2: (A) Axial T1-weighted fast spin-echo and (B) axial STIR. (C) Axial T2-wighted fast spin-echo watersuppressed image and (D) dynamic contrast-enhanced T1-weighted imaging showing double-lumen implants with the inner lumen filled with saline, and the external lumen filled with silicone gel. The right implant is anterior to the pectoralis major muscle (subglandular), and the left implant is posterior to the pectoralis major muscle (subpectoral). This patient underwent a left mastectomy and right adenectomy, with bilateral breast reconstruction.
Figure 4: (A) Axial T1-weighted fast spin-echo and (B) axial T2-weighted fast spin-echo water-suppressed image showing double-lumen implants with the inner lumen filled with saline, and the external lumen filled with silicone gel. Both implants are posterior to the pectoralis major muscle (subfascial or subpectoral plane).
Normal Aspect of Implants IN MRI
Once implanted in the human body, the immune system recognizes the elastomer as a foreign material and responds with an inflammatory process that results in a fibrous capsule [2].
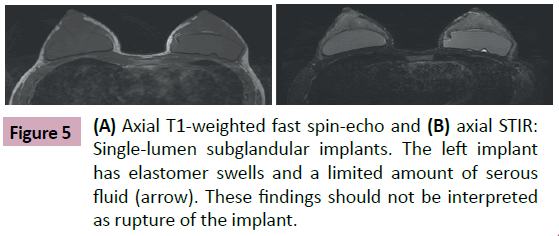
The fibrous capsule can be characterized on the MRI as a thin low signal line on all pulse sequences. It interposes itself between the elastomer and the breast parenchyma or between the elastomer and the pectoralis major muscle depending on the implant location. In asymptomatic patients—especially in cases of early postoperative examination—we have observed that the capsule may suffer a discrete symmetrical signal enhancement. This is the result of the inflammatory response generated by the elastomer and should not be interpreted as a rejection or infection of the implant.
Another frequent and usual finding is the presence of a small amount of serous fluid located inside the fibrous capsule that outlines the implant. This is called a periprosthesis collection (Figure 5). This peri-implant liquid deposition can be symmetrical or asymmetrical. It usually does not determine morphological changes in the elastomer. It is a normal finding with no clinical significance [1-3,7-9].
Implants may also have dimples in their outline due to their accommodation to the restricted space to which they are confined. In some cases, these folds can be deep and simulate the appearance of a shell rupture. These folds are called radial bands [1,2]. On MRI, we can visualize the continuity of these bands in sequential planes of the exam as well as the absence of silicone outside of the implant shell. This is an accurate exam that excludes intracapsular rupture as suspected in other imaging modalities. Large or over-expanded implants nearly always show radial bands on MRI [2].
Integrity Assessment of Implants and Fibrous Capsule
Studies comparing imaging methods show that MRI is the preferable technique to assess implant integrity with 80-90% sensitivity and 90-97% specificity. These metrics help document more precisely the extent of the silicone leakage when present [1-3,7,9,11,12]
Newer generation implants have a durability of 15 to 25 years after implantation with estimated rupture rates of 2% at 5 years and 15-17% at 10 years. Intracapsular ruptures are significantly more common reaching 77-89% of cases depending on the reported series [1,2,7,13].
MRI assessment of implant integrity consists of the characterization of the elastomer shell and fibrous capsule continuity. These are the basic parameters to identify intracapsular or extra capsular ruptures and focal bulging of the implants.
We can diagnose an intracapsular rupture in the case of rupture of the elastomer without discontinuity signs of the fibrous capsule [1,2,6,8]. In the presence of intracapsular rupture, some expected signs on the MRI confirm the diagnosis:
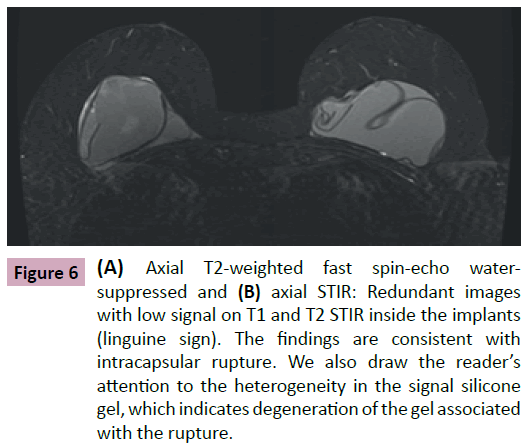
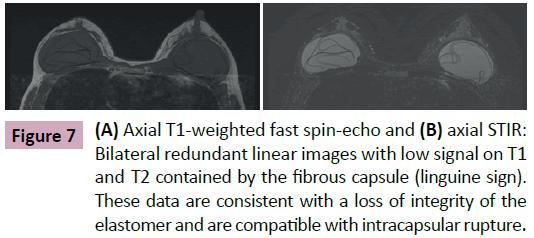
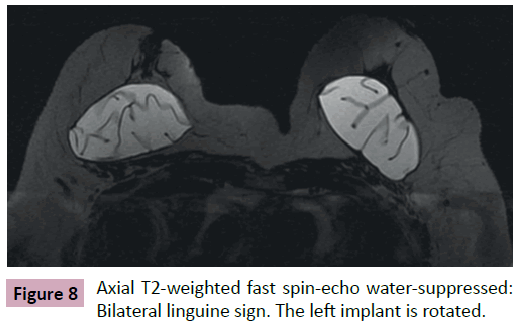
• Linguine signal: linear images with low intensity signal on all sequences, reeled or parallel to each other that are located within the area limited by the fibrous capsule (Figures 6-8).
Figure 6: (A) Axial T2-weighted fast spin-echo watersuppressed and (B) axial STIR: Redundant images with low signal on T1 and T2 STIR inside the implants (linguine sign). The findings are consistent with intracapsular rupture. We also draw the reader’s attention to the heterogeneity in the signal silicone gel, which indicates degeneration of the gel associated with the rupture.
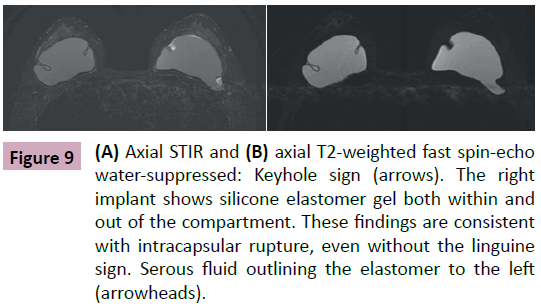
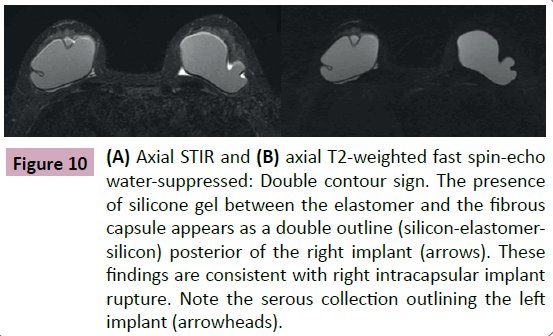
• Keyhole and the double contour signals: In some situations, the rupture of the elastomer is focal or even subtle. The linguine signal may not be present even with an established intracapsular rupture. In these cases, the radiologist will observe the presence of silicone gel between the elastomer and the fibrous capsule mustering a double outline (silicon - elastomer - silicon). This space is normally virtual and in some situations may contain a thin layer of serous liquid [1,2,4,8]. The presence of silicone in this space has no differential diagnosis and is compatible with an intracapsular rupture (Figures 9 and 10).
Figure 9: (A) Axial STIR and (B) axial T2-weighted fast spin-echo water-suppressed: Keyhole sign (arrows). The right implant shows silicone elastomer gel both within and out of the compartment. These findings are consistent with intracapsular rupture, even without the linguine sign. Serous fluid outlining the elastomer to the left (arrowheads).
Figure 10: (A) Axial STIR and (B) axial T2-weighted fast spin-echo water-suppressed: Double contour sign. The presence of silicone gel between the elastomer and the fibrous capsule appears as a double outline (silicon-elastomersilicon) posterior of the right implant (arrows). These findings are consistent with right intracapsular implant rupture. Note the serous collection outlining the left implant (arrowheads).
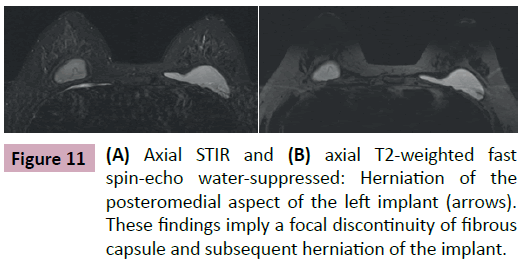
On rare occasions, we see a focal disruption of the fibrous capsule on MRI with no associated signs of intracapsular rupture of the implant. The shell is intact and herniates through the capsule breach causing significant cosmetic changes to the breast. This radiological condition is called focal bulge (Figure 11) [1,4,8,11].
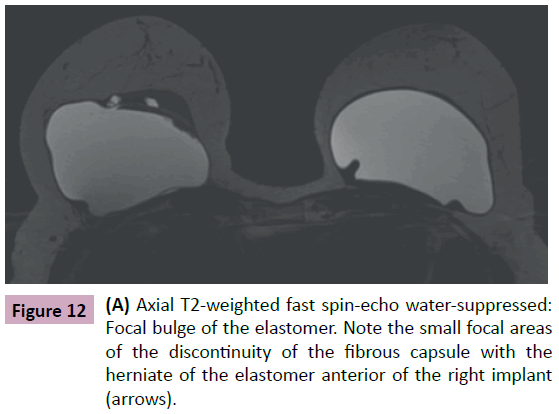
Finally, in extra capsular rupture, we see a concomitant rupture of both the elastomer and the fibrous capsule with silicone gel leakage into the adjacent mammary parenchyma [1,4,8,11]. We emphasize that in both focal bulge and extra-capsular rupture there is a discontinuity in the fibrous capsule; however, the implant shell remains intact in cases of focal bulging (Figure 12).
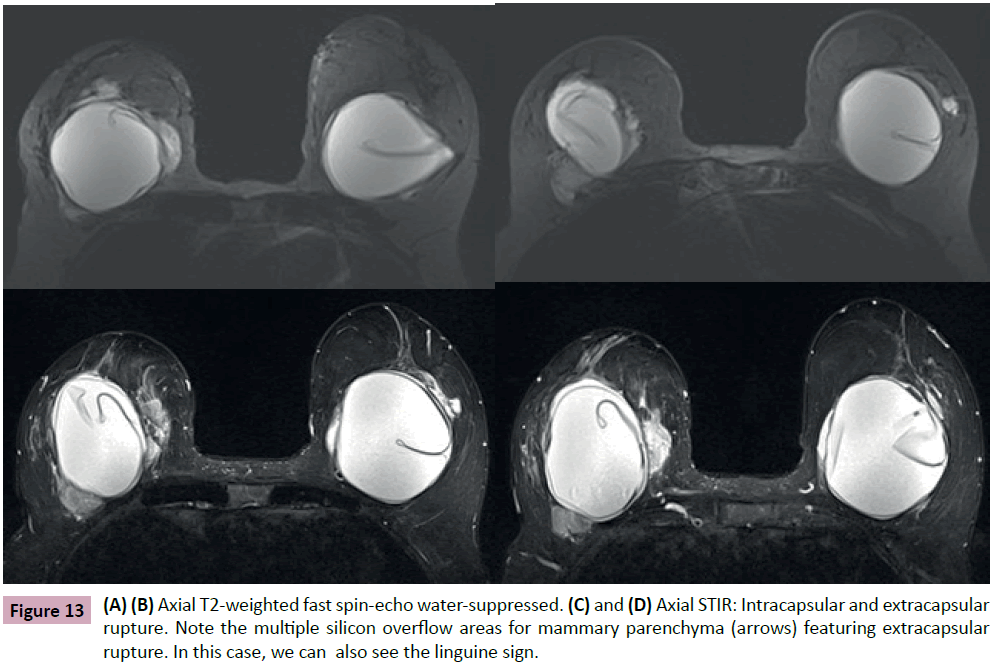
In the case of extra capsular rupture, MRI can use specific sequences to characterize silicone. This can be used to identify leakage/migration of gel to regional lymph nodes, chest wall, pleura, arm, forearm, hand and eventually cases of distant migration to the abdominal wall, liver and inguinal area (Figure 13) [14].
Another rare and poorly understood situation is a systemic leakage of silicone through intact shell and capsule on patients with no history of previous ruptures. This phenomenon is called "bleeding" (Figure 14). As more cohesive gel has been developed the frequency of these occasional findings has decreased [7].
Figure 14: (A)and(B) Axial T2-weighted fast spin-echo watersuppressed: Bleeding. Single-lumen silicone gel implants, which are intact. Silicone migration is observed for the posteromedial aspect of the right breast in the absence of rupture (arrow). The patient had no history of previous ruptures, nor was she injected with silicone-free breasts for cosmetic purposes.
Capsular Contracture and Capsulitis
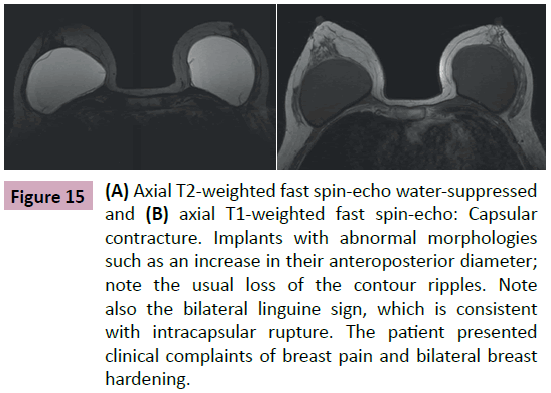
The formation of the fibrous capsule is part of the normal immune response to the implanted elastomer—it usually has no clinical manifestations. In some situations, the capsule can become thicker and harder. This determines cosmetic changes to the breast and is referred to as capsular contracture (Figure 15) [1-12].
Figure 15: (A) Axial T2-weighted fast spin-echo water-suppressed and (B) axial T1-weighted fast spin-echo: Capsular contracture. Implants with abnormal morphologies such as an increase in their anteroposterior diameter; note the usual loss of the contour ripples. Note also the bilateral linguine sign, which is consistent with intracapsular rupture. The patient presented clinical complaints of breast pain and bilateral breast hardening.
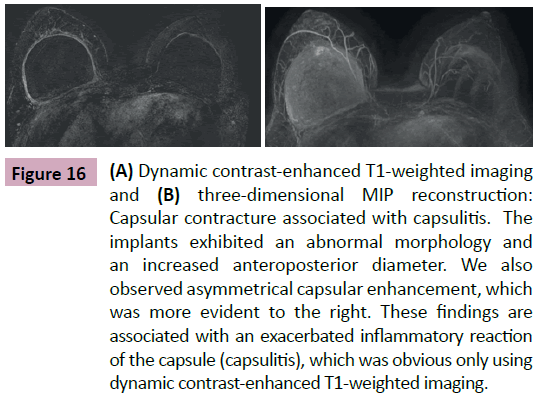
Although the diagnosis of contracture can be established by physical examination, MRI can illustrate an implant with a smooth outline including the loss of their usual swells as well as an increase in its anteroposterior diameter. This gives it an oval appearance. We also observed an asymmetric and disproportionate signal from the fibrous capsule showing an exaggerated inflammatory process. In our department, we use the term capsulitis to identify these findings (Figure 16). In many cases, it is the only radiological finding that confirms capsular contracture, and it is predictive of the degree of inflammation on the fibrous capsule. This signal can predict the potential intraoperative difficulties in replacing the implant. In other cases, capsular thickening is seen without asymmetric enhancement.
Figure 16: (A) Dynamic contrast-enhanced T1-weighted imaging and (B) three-dimensional MIP reconstruction: Capsular contracture associated with capsulitis. The implants exhibited an abnormal morphology and an increased anteroposterior diameter. We also observed asymmetrical capsular enhancement, which was more evident to the right. These findings are associated with an exacerbated inflammatory reaction of the capsule (capsulitis), which was obvious only using dynamic contrast-enhanced T1-weighted imaging.
Water Droplets (Heterogeneity of the Silicone Gel)
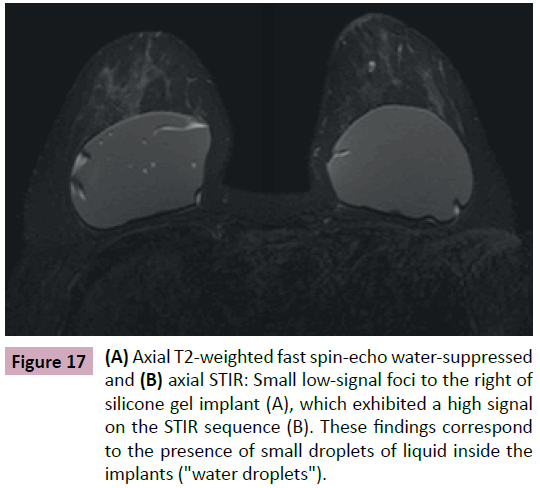
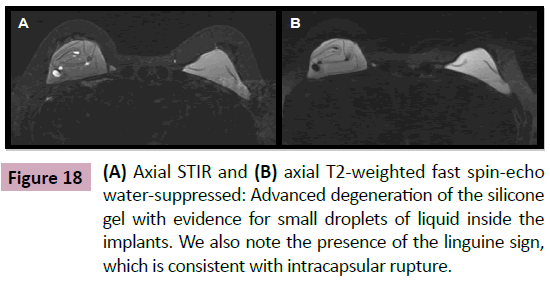
The signal observed in MRI is due to the presence of small droplets of liquid inside the implants (Figure 17). This finding is seen as heterogeneity in the gel especially in cases of intact longstanding implants or in cases of confirmed shell rupture (Figure 18). Their presence is inferred as a gel degeneration process, and it should be included on the medical MRI report.
Figure 17: (A) Axial T2-weighted fast spin-echo water-suppressed and (B) axial STIR: Small low-signal foci to the right of silicone gel implant (A), which exhibited a high signal on the STIR sequence (B). These findings correspond to the presence of small droplets of liquid inside the implants ("water droplets").
Peri-Implant Collection (Encapsulation)
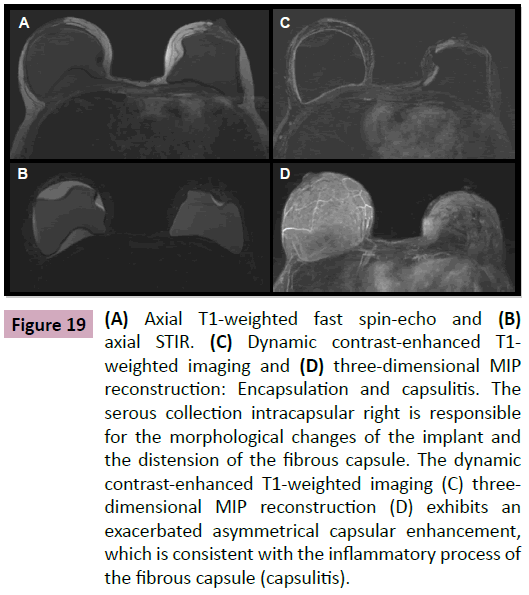
In studying non-complicated implants, we observe thin layers of serous fluid between the elastomer and the fibrous capsule (Figure 19). These collections can be symmetrical or asymmetrical. Generally, they do not determine morphological alterations of the implant, and they are not associated with significant clinical complaints.
Figure 19: (A) Axial T1-weighted fast spin-echo and (B) axial STIR. (C) Dynamic contrast-enhanced T1- weighted imaging and (D) three-dimensional MIP reconstruction: Encapsulation and capsulitis. The serous collection intracapsular right is responsible for the morphological changes of the implant and the distension of the fibrous capsule. The dynamic contrast-enhanced T1-weighted imaging (C) threedimensional MIP reconstruction (D) exhibits an exacerbated asymmetrical capsular enhancement, which is consistent with the inflammatory process of the fibrous capsule (capsulitis).
However, in infectious processes, there is an exacerbated inflammatory reaction of the capsule (capsulitis) or even some kind of direct trauma or intense physical effort. Here, we can see serous, hematic or purulent collections around the implants.
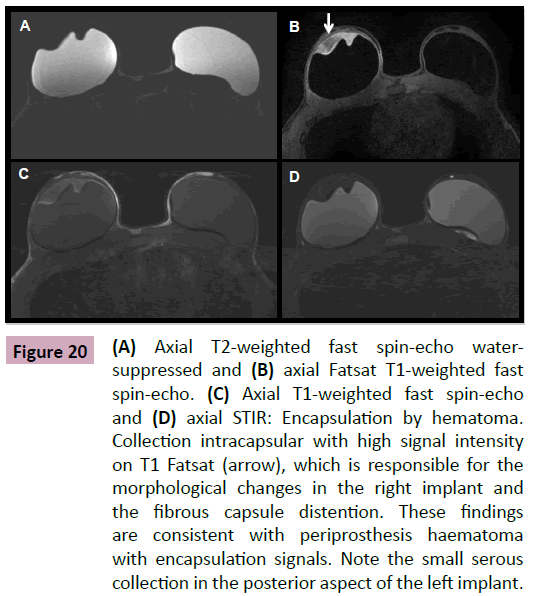
We stress the importance of using T1- and T2-weighted sequences with selective fat saturation signal as well as the intravenous injection of paramagnetic contrast. This is associated with the implant assessment protocol and allows the radiologist to differentiate the type of collection (hematic versus serous) (Figure 20). It also provides information regarding the presence of inflammation on the fibrous capsule and possible complications such as edema, inflammation, or infection of the mammary parenchyma. In addition, the use of contrast agents increases the accuracy of the method in the detection of breast cancer that does not appear in other exams.
Figure 20: (A) Axial T2-weighted fast spin-echo watersuppressed and (B) axial Fatsat T1-weighted fast spin-echo. (C) Axial T1-weighted fast spin-echo and (D) axial STIR: Encapsulation by hematoma. Collection intracapsular with high signal intensity on T1 Fatsat (arrow), which is responsible for the morphological changes in the right implant and the fibrous capsule distention. These findings are consistent with periprosthesis haematoma with encapsulation signals. Note the small serous collection in the posterior aspect of the left implant.
Intramammary Silicon Injection
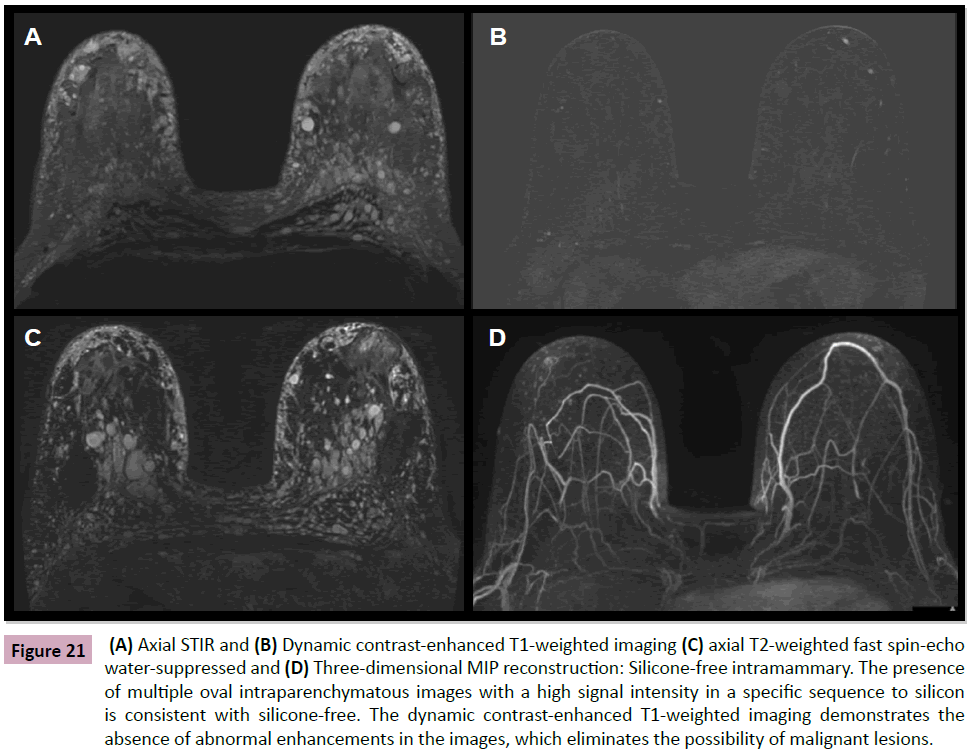
With surprising frequency, paraffin, liquid silicone or other substances have been injected into breasts by patients and/or non-medical professionals (Figure 21) [15]. The injected silicone is not firm and usually not palpable, but it can cause an intense inflammatory reaction that may evolve into a hard granuloma that simulates malignant nodules on clinical examination, mammography and/or ultrasonography. MRI is the only imaging method that can differentiate a malignant nodule from a silicone granuloma (Figure 22). This is done via specific sequences to characterize the material inside these nodules. Silicone shows either no signal or a pattern of delayed and progressive enhancement inside the silicone granuloma in dynamic sequences with intravenous infusion of paramagnetic contrast (Figures 23 and 24) [15,16].
Figure 21: (A) Axial STIR and (B) Dynamic contrast-enhanced T1-weighted imaging (C) axial T2-weighted fast spin-echo water-suppressed and (D) Three-dimensional MIP reconstruction: Silicone-free intramammary. The presence of multiple oval intraparenchymatous images with a high signal intensity in a specific sequence to silicon is consistent with silicone-free. The dynamic contrast-enhanced T1-weighted imaging demonstrates the absence of abnormal enhancements in the images, which eliminates the possibility of malignant lesions.
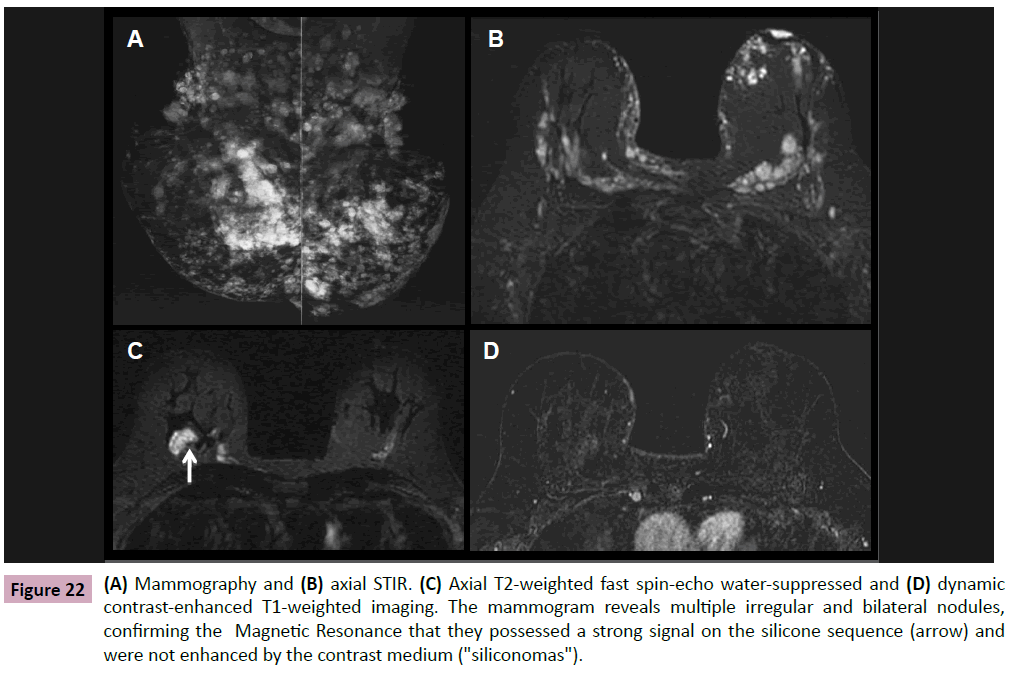
Figure 22: (A) Mammography and (B) axial STIR. (C) Axial T2-weighted fast spin-echo water-suppressed and (D) dynamic contrast-enhanced T1-weighted imaging. The mammogram reveals multiple irregular and bilateral nodules, confirming the Magnetic Resonance that they possessed a strong signal on the silicone sequence (arrow) and were not enhanced by the contrast medium ("siliconomas").
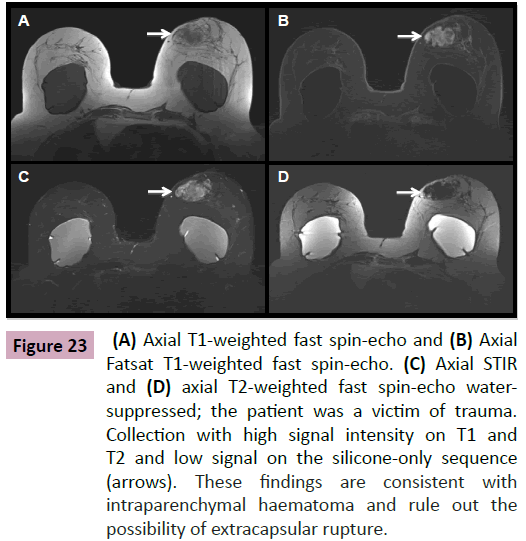
Figure 23: (A) Axial T1-weighted fast spin-echo and (B) Axial Fatsat T1-weighted fast spin-echo. (C) Axial STIR and (D) axial T2-weighted fast spin-echo watersuppressed; the patient was a victim of trauma. Collection with high signal intensity on T1 and T2 and low signal on the silicone-only sequence (arrows). These findings are consistent with intraparenchymal haematoma and rule out the possibility of extracapsular rupture.
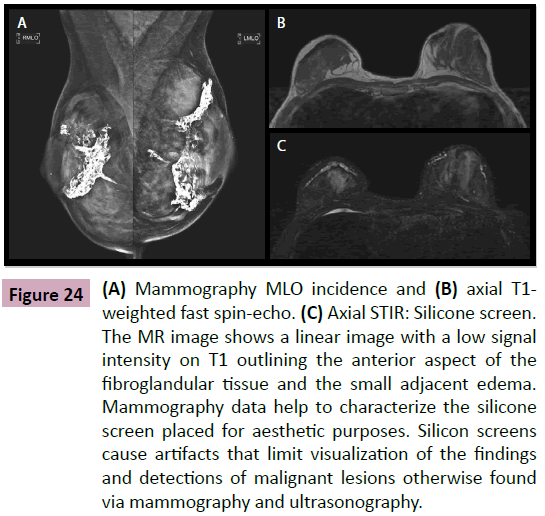
Figure 24: (A) Mammography MLO incidence and (B) axial T1- weighted fast spin-echo. (C) Axial STIR: Silicone screen. The MR image shows a linear image with a low signal intensity on T1 outlining the anterior aspect of the fibroglandular tissue and the small adjacent edema. Mammography data help to characterize the silicone screen placed for aesthetic purposes. Silicon screens cause artifacts that limit visualization of the findings and detections of malignant lesions otherwise found via mammography and ultrasonography.
Cancer and Silicone
There is currently no published evidence demonstrating an increased risk of breast cancer in patients with silicone implants. Published data regarding age and survival in patients with breast implants who develop cancer are similar to the general population [6,17,18]. Some studies suggest that the capsule vascularization may favor tumor growth if it occurs [19,20]. Women who have breast reconstruction with implants after breast cancer surgery have survival rates similar implant-free women; there is no contraindication against their use (Figure 25) [11].
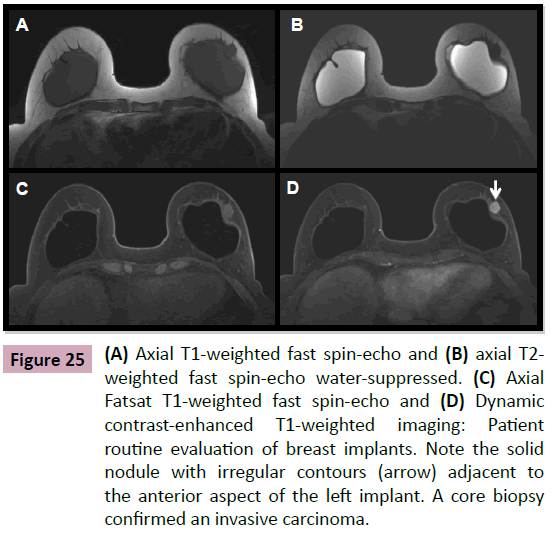
Figure 25: (A) Axial T1-weighted fast spin-echo and (B) axial T2- weighted fast spin-echo water-suppressed. (C) Axial Fatsat T1-weighted fast spin-echo and (D) Dynamic contrast-enhanced T1-weighted imaging: Patient routine evaluation of breast implants. Note the solid nodule with irregular contours (arrow) adjacent to the anterior aspect of the left implant. A core biopsy confirmed an invasive carcinoma.
References
- Prado Monzo C, Mallo Alonso MR, Vieito Fuentes JM, Arias Gonzales M, Vigo ES (2013) Imaging in breast implants: Normal and pathological findings in mammography, ultrasound and MRI. European Society of Radiology: 21-28.
- Middleton MS (2014) MR Evaluation of Breast Implants. Radiol Clin N Am 52: 591–608.
- Brenner J (2013) Evaluation of Breast Silicone Implants. Magn Reson Imaging Clin N Am 21: 547–560.
- Shah M, Tanna N, Margolies L (2014) Magnetic resonance imaging of breast implants. Magn Reson Imaging 23: 345-353
- Kim SH, Lipson JA, Moran CJ, Shimakawa A, Kuo J, et al. (2013) Image quality and diagnostic performance of silicone-specific breast MRI. Magnetic Resonance Imaging 31: 1472–1478.
- Yang N, Muradali D (2011) The augmented breast: A pictorial review of the abnormal and unusual. AJR: 196.
- Rebecca Roller, Alison Chetlen, Claudia Kasales (2014) Imaging of breast implants and their associated complications. J Am Osteopath Coll Radiol. 3.
- American College of Radiology (2013) Breast imaging reporting and data system. Breast imaging atlas. 5th ed. Reston, Va: American College of Radiology
- Holmich LR, Vejborg I, Conrad C (2005) The diagnosis of breast implant rupture: MRI findings compared with findings at explantation. Eur J Radiol. 53: 213-225.
- Sardanelli F (2010) Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer 46: 1296–1316.
- Juanpere S, Perez E, Huc O (2011) Imaging of breast implants-a pictorial review. Insights Imaging 2: 653-670.
- Di Benedetto G, Cecchini S, Grassetti L (2008) Comparative study of breast implant rupture using mammography, sonography, and magnetic resonance imaging: Correlation with surgical findings. The Breast Journal 14: 532-537.
- Brown SL, Middleton MS, Berg WA (2000) Prevalence of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama. AJR Am J Roentgenol 175: 1057- 1064.
- Po J, Margolis DJA, Cunningham CH, Herfkens RJ, Ikeda DM, et al. (2006) Water-selective spectral–spatial contrast-enhanced breast MRI for cancer detection in patients with extracapsular and injected free silicone. Magnetic Resonance Imaging 24: 1363–1367.
- Aktouf A, Auquit-Auckbur L, Coquerel-Beghin D, Delpierre V, Milliez P Y (2012) Breast augmentation by Poly Implant Prothe`ses silicone implants: Retrospective study about 99 patients. Rupture analysis and management. Annales de chirurgie plastique esthétique 57: 558-566.
- Brown S L, Silverman B G, Berg W A (1997) Rupture of silicone-gel breast implants: Causes, sequelae, and diagnosis. Lancet 350: 1531– 1537.
- Tokin CA, Wallace AM (2012) Breast cancer presenting within or adjacent to the breast implant capsule: A case series and clinical recommendations. Clinical Breast Cancer 12: 296-299.
- Bryant H, Brasher P (1995) Breast implants and breast cancer: Reanalysis of a linkage study. N Engl J Med 332: 1535–1539.
- Mason AC, White CS, Mcavoy MA, Golberg N (1995) MR Imaging of slipped stacked breast implants: A potential pitfall in the diagnosis of intracapsular rupture. Magnetic Resonance Imaging, 12: 339-342.
- Champaneria MC, Wong WW, Hill ME, Gupta SC (2012) The evolution of breast reconstruction: A historical perspective. World J Surg. 36: 730-742.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences